Tissue Engineering (INGT)
Next, there are some guidelines for medical and individualized cases with their coding, obtained after analyzing the respective references, and grouped by case study and common trends, highlighting in bold the competitive advantages that can become innovations.
INGT-01. The external geometry of the scaffold must correspond to that of the tissue to be implanted and extracted using imaging (tomography, resonance, 3D scanning) [1]–[4].
INGT-02. The internal structure must have a resistance similar to that of the surrounding tissues in case of implant and a minimum resistance to preserve its structural integrity [1]–[4].
INGT-03. Customizing the internal pattern and combining it with an external shape according to tissue can be automated to a certain extent generating a radical innovation that supports the design process, streamlines the whole process, and reduces costs at the design stage [3], [4].
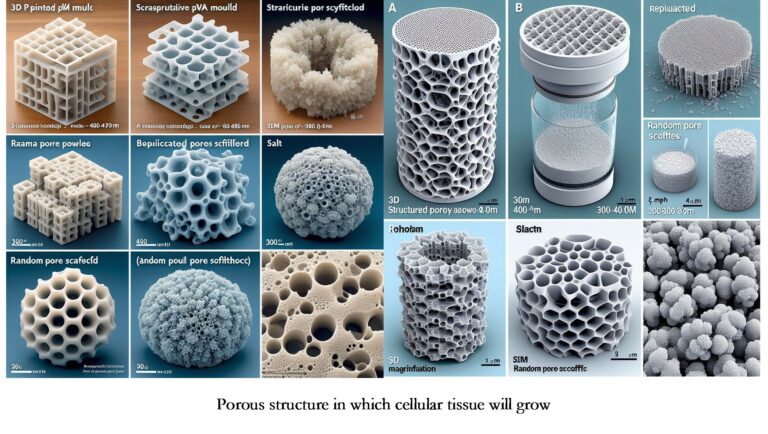
INGT-04. As for pore sizes, use 250 μm or more because it favors the growth of blood vessels. Based on this criterion, sizes of 300 μm to 600 μm of salt crystals in indirect dissolution fabrication of PVA are feasible, and filament spacing of 800 μm to 850 μm has proven effective [4].
INGT-05. Fabricate Tissue scaffolds directly or indirectly. A case of the indirect way: positive mold fabricated with FFF, PVA material, water, and salt grains. Subsequently, PDMS and water sacrifice the PVA [4].
INGT-06. Regarding timing, cells were seeded on the scaffold, cultured in vitro for three months in the case of ear implants, and cultured with chondrocytes for 2 and 4 weeks before implantation to produce a tissue-engineered trachea [1],[2].
INGT-07. Materials include:
- Absorbable materials like PCL, PLA, and PEG.
- Non-absorbable materials such as polyurethane or polytetrafluoroethylene (PTFE).
- Commercially available cellular scaffolds like Alloderm DermACELL, FlexHD, or Integra have proven effective for wound healing.
- For specific applications:
- Ear scaffolds use 3D printed polycaprolactone mesh wrapped with PGA nonwoven fibers and coated with PLA.
- PCL scaffolds, cultured with chondrocytes, replace rabbit trachea.
- In SLS, maintaining hydroxyapatite (HA) at or below 40 wt% ensures structural integrity in polyetheretherketone/hydroxyapatite (PEEK/HA).
- In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be kept at or below 30 wt% for satisfactory scaffold samples with well-defined pore interconnectivity and structural integrity.
PCL is commonly used in FDM processes [1]–[4]
Please refer to the original bibliographic references or consult the References database or Medical database for more details.
References
[1] D. M. Devine, Ed., Polymer-Based Additive Manufacturing. Springer International Publishing, 2019.
[2] A. Foerster, L. R. Cantu, R. Wildman, and C. Tuck, “Current Market for Biomedical Implants,” in Polymer-Based Additive Manufacturing, Springer International Publishing, 2019, pp. 97–119.
[3] J. An, J. E. M. Teoh, R. Suntornnond, and C. K. Chua, “Design and 3D Printing of Scaffolds and Tissues,” Engineering, vol. 1, no. 2, pp. 261–268, Jun. 2015.
[4] S. Mohanty et al., “Fabrication of scalable tissue engineering scaffolds with dual-pore microarchitecture by combining 3D printing and particle leaching,” Mater. Sci. Eng. C, vol. 61, pp. 180–189, 2016.
