Database for Medical and personalization applications.
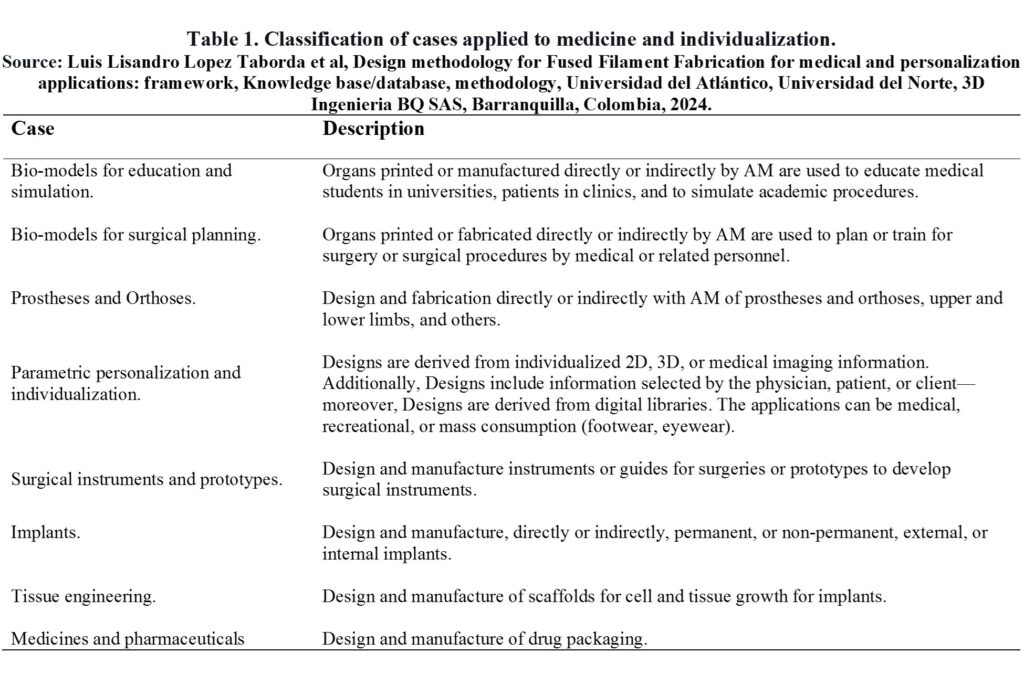
Table 1 summarizes the classification and description of the cases applied to medicine and individualization found during the documentary review’s analysis.
Figure 1 summarizes the medical case subclassifications and individualization that were obtained.
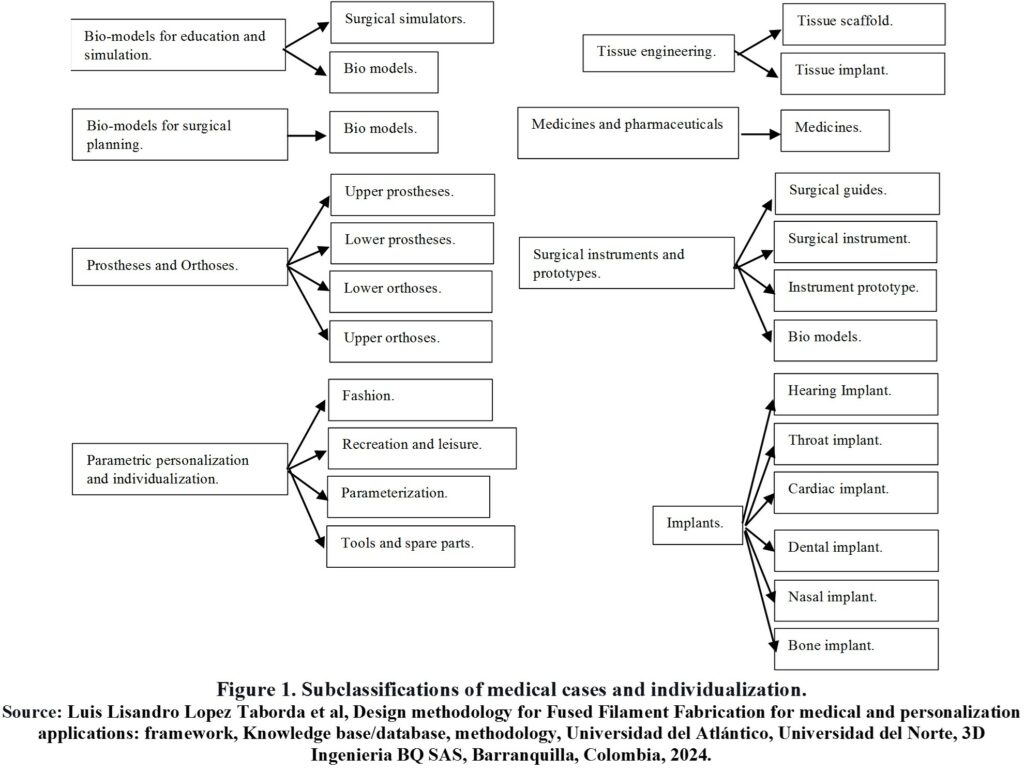
Figure 2 summarizes the main headings of the database.
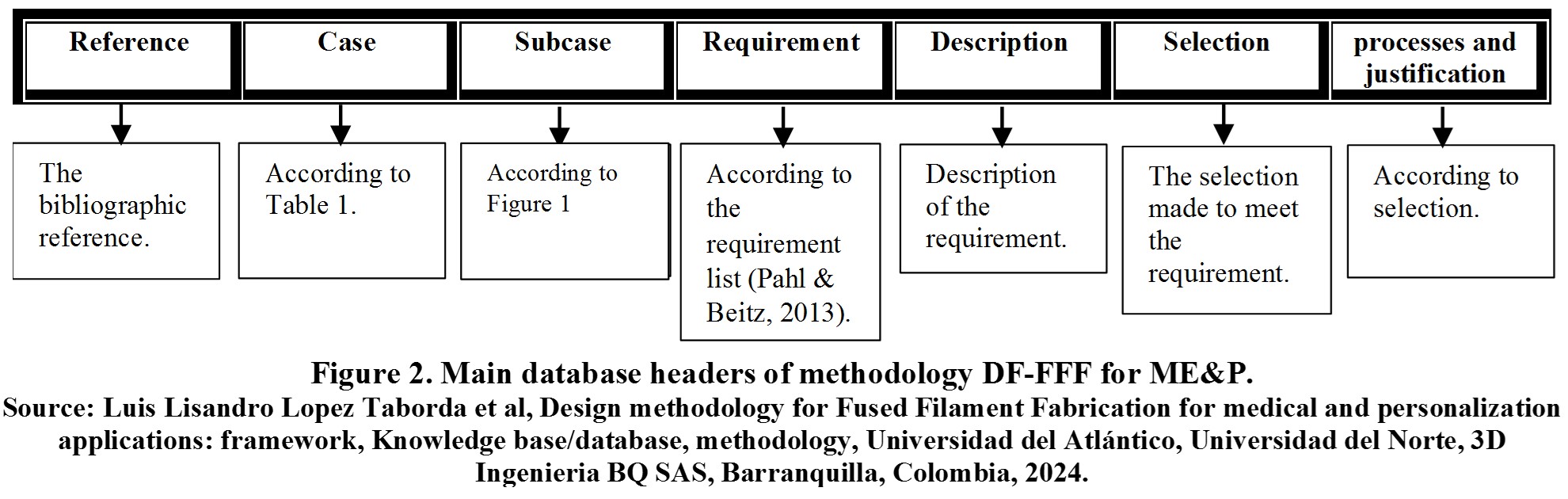
Below is a detailed explanation of each heading in Figure 2.
- Reference: Contains the bibliographic reference analyzed to generate specific and general guidelines.
- Case:One (1) of the eight (8) cases in Table 1 in which the analyzed reference can be classified.
- Subcase:Some of the specific products or sub-cases in Figure 1, depending on the case to which the reference belongs.
- Requirement: It is a demand, obligation, or desirable quality to be met by the product. The product has more than one, and it is defined from the analysis of the reference and requirement lists in the specialized bibliography [1], [2], among others.
- Description: Description of each requirement of a specific product or analyzed case.
- Selection:It is a decision or selection of a product specification to meet a requirement.
- Process and justification: Process using which the product specification is defined.
Under each heading or column is the respective information, and each row contains information related to a specific case or reference. The purpose is to search according to the desired case to apply the guidelines of other cases to one’s case. In case of detailed information, the reference can be consulted directly.
Consult the database of medical cases and personalization below [3]-[4].
Medical & Personalization
| Group | case | subcase | References | objective | Method | Results | Conclusions | requirement | Description | Selection | Process/ Justification/ Result |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GME1 | Biomodels for education and simulation | Surgical simulators | ME6 | Design, Manufacture with 3D printing, and test a Laparoscopic Surgery Simulator for the Common Bile Duct. | Digital images of hepatic anatomy and standard dimensions of a laparoscopic trainer were used to create a beginner-level laparoscopic choledochal surgery model. The design was manufactured using a Systems 660pro machine (SLS) with VisiJet PXL powder to create a liver mold. This included a cuboid portal in which disposable hybrid components representing the hepatic and pancreatic ducts and the choledochal cyst were fitted. The mold was used to create soft silicone replicas with T28 resin and T5 fast catalyst. The model was evaluated on a national pediatric surgery training day. | The 10 delegates who tested the simulation felt that the tactile similarity was good (5.6/10 ± 1.71, 10 = like the real thing), it was not too complex (6.2/10 ± 1.35; where 1 = too simple, 10 = too complicated), and overall very useful (7.36/10 ± 1.57, 10 = invaluable). 100% stated that they felt they could reproduce this in their own centers, and 100% would recommend this simulation to their colleagues. | Although this first phase of simulation of excision of choledochal cysts requires further development, 3D printing provides a useful means to create specific and detailed simulations for rare and complex operations with great potential for development. | Simulator material | The simulator material must resemble the real tissues that will be intervened | Soft silicone with T28 resin and fast T5 catalyst (1:20 catalyst:silicone). | Soft material that simulates soft tissues |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME6 | Design, Manufacture with 3D printing, and test a Laparoscopic Surgery Simulator for the Common Bile Duct. | Digital images of hepatic anatomy and standard dimensions of a laparoscopic trainer were used to create a beginner-level laparoscopic choledochal surgery model. The design was manufactured using a Systems 660pro machine (SLS) with VisiJet PXL powder to create a liver mold. This included a cuboid portal in which disposable hybrid components representing the hepatic and pancreatic ducts and the choledochal cyst were fitted. The mold was used to create soft silicone replicas with T28 resin and T5 fast catalyst. The model was evaluated on a national pediatric surgery training day. | The 10 delegates who tested the simulation felt that the tactile similarity was good (5.6/10 ± 1.71, 10 = like the real thing), it was not too complex (6.2/10 ± 1.35; where 1 = too simple, 10 = too complicated), and overall very useful (7.36/10 ± 1.57, 10 = invaluable). 100% stated that they felt they could reproduce this in their own centers, and 100% would recommend this simulation to their colleagues. | Although this first phase of simulation of excision of choledochal cysts requires further development, 3D printing provides a useful means to create specific and detailed simulations for rare and complex operations with great potential for development. | Simulator insert material | The cuboid portal was to be filled with a disposable and reusable simulated bile tree: The simulator material must resemble the hardness of the real tissues to be operated on. | This included a square sponge with an embedded balloon inside, a surgical glove finger to simulate dilated proximity ducts, and an electrical cable insulation tube to represent the bile and common pancreatic ducts (conventional materials). | Ability to reuse the simulator, reduce the cost of the simulator, Soft material that simulates soft tissues |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME6 | Design, Manufacture with 3D printing, and test a Laparoscopic Surgery Simulator for the Common Bile Duct. | Digital images of hepatic anatomy and standard dimensions of a laparoscopic trainer were used to create a beginner-level laparoscopic choledochal surgery model. The design was manufactured using a Systems 660pro machine (SLS) with VisiJet PXL powder to create a liver mold. This included a cuboid portal in which disposable hybrid components representing the hepatic and pancreatic ducts and the choledochal cyst were fitted. The mold was used to create soft silicone replicas with T28 resin and T5 fast catalyst. The model was evaluated on a national pediatric surgery training day. | The 10 delegates who tested the simulation felt that the tactile similarity was good (5.6/10 ± 1.71, 10 = like the real thing), it was not too complex (6.2/10 ± 1.35; where 1 = too simple, 10 = too complicated), and overall very useful (7.36/10 ± 1.57, 10 = invaluable). 100% stated that they felt they could reproduce this in their own centers, and 100% would recommend this simulation to their colleagues. | Although this first phase of simulation of excision of choledochal cysts requires further development, 3D printing provides a useful means to create specific and detailed simulations for rare and complex operations with great potential for development. | Insert simulator geometry | The cuboid portal should be filled with a disposable and reusable simulated bile tree: The geometry should be as similar as possible to that of a real and average patient. | This included a square sponge with an embedded balloon inside, a surgical glove finger to simulate dilated proximity ducts, and an electrical cable insulation tube to represent the bile and common pancreatic ducts (conventional materials). | Ability to reuse the simulator, reduce the simulator |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME6 | Design, Manufacture with 3D printing, and test a Laparoscopic Surgery Simulator for the Common Bile Duct. | Digital images of hepatic anatomy and standard dimensions of a laparoscopic trainer were used to create a beginner-level laparoscopic choledochal surgery model. The design was manufactured using a Systems 660pro machine (SLS) with VisiJet PXL powder to create a liver mold. This included a cuboid portal in which disposable hybrid components representing the hepatic and pancreatic ducts and the choledochal cyst were fitted. The mold was used to create soft silicone replicas with T28 resin and T5 fast catalyst. The model was evaluated on a national pediatric surgery training day. | The 10 delegates who tested the simulation felt that the tactile similarity was good (5.6/10 ± 1.71, 10 = like the real thing), it was not too complex (6.2/10 ± 1.35; where 1 = too simple, 10 = too complicated), and overall very useful (7.36/10 ± 1.57, 10 = invaluable). 100% stated that they felt they could reproduce this in their own centers, and 100% would recommend this simulation to their colleagues. | Although this first phase of simulation of excision of choledochal cysts requires further development, 3D printing provides a useful means to create specific and detailed simulations for rare and complex operations with great potential for development. | Simulator Geometry | The geometry should be as close as possible to that of a real and average patient. | The translated value of the provided data Se emplearon imágenes hepáticas y dimensiones de simuladores estándar in English is Liver images and standard simulator dimensions were used. | Simulate the real experience |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME6 | Design, Manufacture with 3D printing, and test a Laparoscopic Surgery Simulator for the Common Bile Duct. | Digital images of hepatic anatomy and standard dimensions of a laparoscopic trainer were used to create a beginner-level laparoscopic choledochal surgery model. The design was manufactured using a Systems 660pro machine (SLS) with VisiJet PXL powder to create a liver mold. This included a cuboid portal in which disposable hybrid components representing the hepatic and pancreatic ducts and the choledochal cyst were fitted. The mold was used to create soft silicone replicas with T28 resin and T5 fast catalyst. The model was evaluated on a national pediatric surgery training day. | The 10 delegates who tested the simulation felt that the tactile similarity was good (5.6/10 ± 1.71, 10 = like the real thing), it was not too complex (6.2/10 ± 1.35; where 1 = too simple, 10 = too complicated), and overall very useful (7.36/10 ± 1.57, 10 = invaluable). 100% stated that they felt they could reproduce this in their own centers, and 100% would recommend this simulation to their colleagues. | Although this first phase of simulation of excision of choledochal cysts requires further development, 3D printing provides a useful means to create specific and detailed simulations for rare and complex operations with great potential for development. | Geometry of the Mold | The geometry should be as close as possible to that of a real and average patient. | Rectangular mold with external dimensions of standard simulator. | Simulate the real experience |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME6 | Design, Manufacture with 3D printing, and test a Laparoscopic Surgery Simulator for the Common Bile Duct. | Digital images of hepatic anatomy and standard dimensions of a laparoscopic trainer were used to create a beginner-level laparoscopic choledochal surgery model. The design was manufactured using a Systems 660pro machine (SLS) with VisiJet PXL powder to create a liver mold. This included a cuboid portal in which disposable hybrid components representing the hepatic and pancreatic ducts and the choledochal cyst were fitted. The mold was used to create soft silicone replicas with T28 resin and T5 fast catalyst. The model was evaluated on a national pediatric surgery training day. | The 10 delegates who tested the simulation felt that the tactile similarity was good (5.6/10 ± 1.71, 10 = like the real thing), it was not too complex (6.2/10 ± 1.35; where 1 = too simple, 10 = too complicated), and overall very useful (7.36/10 ± 1.57, 10 = invaluable). 100% stated that they felt they could reproduce this in their own centers, and 100% would recommend this simulation to their colleagues. | Although this first phase of simulation of excision of choledochal cysts requires further development, 3D printing provides a useful means to create specific and detailed simulations for rare and complex operations with great potential for development. | Mold Material | The material must guarantee the geometry of the simulator and facilitate demolding. | Nylon powder (visijet pxl), for demolding silicone spray is used Ambersil Formula 5 (manufactured in available laser sintering selective printer, 3D systems project 660pro) | Manufacturing facility |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME6 | Design, Manufacture with 3D printing, and test a Laparoscopic Surgery Simulator for the Common Bile Duct. | Digital images of hepatic anatomy and standard dimensions of a laparoscopic trainer were used to create a beginner-level laparoscopic choledochal surgery model. The design was manufactured using a Systems 660pro machine (SLS) with VisiJet PXL powder to create a liver mold. This included a cuboid portal in which disposable hybrid components representing the hepatic and pancreatic ducts and the choledochal cyst were fitted. The mold was used to create soft silicone replicas with T28 resin and T5 fast catalyst. The model was evaluated on a national pediatric surgery training day. | The 10 delegates who tested the simulation felt that the tactile similarity was good (5.6/10 ± 1.71, 10 = like the real thing), it was not too complex (6.2/10 ± 1.35; where 1 = too simple, 10 = too complicated), and overall very useful (7.36/10 ± 1.57, 10 = invaluable). 100% stated that they felt they could reproduce this in their own centers, and 100% would recommend this simulation to their colleagues. | Although this first phase of simulation of excision of choledochal cysts requires further development, 3D printing provides a useful means to create specific and detailed simulations for rare and complex operations with great potential for development. | Simulator Configuration | It should be complex enough to be useful in training but simple enough to ensure the education and reproducibility of the procedure with the same simulator, and in other centers. | Simulator consisting of two parts, the larger one representing the patient's exterior, and an insert containing a biliary tree that would represent the tissue to be accessed with laparoscopy. | guarantee utility, reduce costs, and increase reproducibility and reusability. |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME6 | Design, Manufacture with 3D printing, and test a Laparoscopic Surgery Simulator for the Common Bile Duct. | Digital images of hepatic anatomy and standard dimensions of a laparoscopic trainer were used to create a beginner-level laparoscopic choledochal surgery model. The design was manufactured using a Systems 660pro machine (SLS) with VisiJet PXL powder to create a liver mold. This included a cuboid portal in which disposable hybrid components representing the hepatic and pancreatic ducts and the choledochal cyst were fitted. The mold was used to create soft silicone replicas with T28 resin and T5 fast catalyst. The model was evaluated on a national pediatric surgery training day. | The 10 delegates who tested the simulation felt that the tactile similarity was good (5.6/10 ± 1.71, 10 = like the real thing), it was not too complex (6.2/10 ± 1.35; where 1 = too simple, 10 = too complicated), and overall very useful (7.36/10 ± 1.57, 10 = invaluable). 100% stated that they felt they could reproduce this in their own centers, and 100% would recommend this simulation to their colleagues. | Although this first phase of simulation of excision of choledochal cysts requires further development, 3D printing provides a useful means to create specific and detailed simulations for rare and complex operations with great potential for development. | Functionality test | Professionals related must test the simulator and provide feedback with their opinion. | Twenty senior pediatric surgical apprentices attended the national competition. training day. The biliary station consisted of two parts: simulated laparoscopic cholecystectomy with porcine organs and simulated choledochal cyst as described. Ten delegates tested the latest simulation. Feedback was collected from the 10. | In visual analog scoring, the simulation obtained an average of 5.6 / 10 (range: 3-8; SD 1.71), where 10 means it feels like the real operation and 1 was not at all the same. Delegates felt that the complexity was scored correctly with an average of 6.2 / 10 (range: 4-8; SD 1.35), where 1 = too simple and 10 = too complicated. The simulation was also considered very useful overall with an average of 7.36 / 10 (range: 4-9; SD 1.57), where 1 = useless and 10 = invaluable. It was considered easily reproducible in their own units and 100% also claimed they would recommend the simulation to their colleagues. |
| GME1 | Biomodels for education and simulation | Biomodels | ME12 | Design and Manufacture with 3D printing, Anatomical models for teaching | They graphically designed 3D meshes or modified imported data from cross-sectional images to develop physical models specifically for teaching complex segments and branches of anatomy. 3D printing itself is easily accessible through online commercial services, and the models are made of polyamide or plaster. | Anatomical models of the liver, lungs, prostate, coronary arteries, and the Circle of Willis were created. These models have advantages that include customizable details, relative low cost, complete control of design focused on subsegments, potential for color coding, and the use of cross-sectional images combined with graphic design. | The radiologists have the opportunity to serve as leaders in medical education and clinical care with 3D printed models that provide beneficial interaction with patients, physicians, and learners in all specialties, proactively assuming the role of educator. Complex models can be developed to demonstrate normal anatomy or common pathology for medical education purposes. There is a need for randomized trials, which radiologists can design, to demonstrate the utility and effectiveness of 3D printed models for teaching simple and complex anatomy, simulating interventions, measuring patient satisfaction, and improving clinical care. | Simulator material | The translated value of El material debe ser durable in English is The material must be durable. | Nylon (manufacturing by remote 3D printing) | Durability of the Material |
| GME1 | Biomodels for education and simulation | Biomodels | ME12 | Design and Manufacture with 3D printing, Anatomical models for teaching | They graphically designed 3D meshes or modified imported data from cross-sectional images to develop physical models specifically for teaching complex segments and branches of anatomy. 3D printing itself is easily accessible through online commercial services, and the models are made of polyamide or plaster. | Anatomical models of the liver, lungs, prostate, coronary arteries, and the Circle of Willis were created. These models have advantages that include customizable details, relative low cost, complete control of design focused on subsegments, potential for color coding, and the use of cross-sectional images combined with graphic design. | The radiologists have the opportunity to serve as leaders in medical education and clinical care with 3D printed models that provide beneficial interaction with patients, physicians, and learners in all specialties, proactively assuming the role of educator. Complex models can be developed to demonstrate normal anatomy or common pathology for medical education purposes. There is a need for randomized trials, which radiologists can design, to demonstrate the utility and effectiveness of 3D printed models for teaching simple and complex anatomy, simulating interventions, measuring patient satisfaction, and improving clinical care. | Simulator Geometry | The geometry must correspond to the anatomy of the specific organ, including vessels, arteries, connections, even the use of colors, voids, and sections (assembly) to better illustrate the anatomical composition. | The pulmonary model: The initial digital 3D mesh is graphically designed and obtained from the online library free of charge (3DCADBrowser.com). Modifications were made using a free version of Autodesk 3D Studio Max with a student/educator license to add the main pulmonary arteries to provide the connection between each lung, as well as to create a surface color map representing the lobes and segments of the lungs. The hepatic model: 2D diagrams were hand-drawn to communicate liver cutting planes. A graphic designer was hired, whose services were available as a freelance artist for an online fee (FlatPyramid.com). Multiple instances of corrections had to be made to achieve the final ideal model. The coronary arterial system was part of a pre-designed cardiopulmonary system commercially available and through an online catalog of professional 3D models (TurboSquid.com). Minor modifications were required using graphic design software (Autodesk 3D Studio Max, San Rafael, CA, USA), including the addition of the septal branches of the left anterior descending artery, as well as increasing the diameter of all vessels. 3D prostate model: It was obtained from a free online source that had already been reconstructed in 3D from MRI using open-source Slicer software (Slicer.org). The posterior anatomical subdivision was performed in Autodesk 3D Studio Max, which requires intermediate-level graphic design skills and considerations on how the components of the final model can fit together in the physical world like puzzle pieces. The overall curvature of the urethra was reduced, and a gradual decrease was applied to the urethral prostate. Note: The translation has been provided in English. | The modifications were made in order to better illustrate the anatomy of the organ to patients or students, according to the criteria of the radiologist or specialist. In some cases, it was done to ensure the physical integrity of the model (minimum wall thickness) or to ensure the assemblability (separation of parts). |
| GME1 | Biomodels for education and simulation | Biomodels | ME12 | Design and Manufacture with 3D printing, Anatomical models for teaching | They graphically designed 3D meshes or modified imported data from cross-sectional images to develop physical models specifically for teaching complex segments and branches of anatomy. 3D printing itself is easily accessible through online commercial services, and the models are made of polyamide or plaster. | Anatomical models of the liver, lungs, prostate, coronary arteries, and the Circle of Willis were created. These models have advantages that include customizable details, relative low cost, complete control of design focused on subsegments, potential for color coding, and the use of cross-sectional images combined with graphic design. | The radiologists have the opportunity to serve as leaders in medical education and clinical care with 3D printed models that provide beneficial interaction with patients, physicians, and learners in all specialties, proactively assuming the role of educator. Complex models can be developed to demonstrate normal anatomy or common pathology for medical education purposes. There is a need for randomized trials, which radiologists can design, to demonstrate the utility and effectiveness of 3D printed models for teaching simple and complex anatomy, simulating interventions, measuring patient satisfaction, and improving clinical care. | Simulator size | The size should facilitate didactics and mechanical resistance but also reduce cost. | The final size was a combination of the original model size plus modification to achieve the required price, and the wall thickness to ensure structural safety of the model. | Combination of numerical cost criteria and manufacturer expertise to define the minimum wall thickness. |
| GME1 | Biomodels for education and simulation | Biomodels | ME12 | Design and Manufacture with 3D printing, Anatomical models for teaching | They graphically designed 3D meshes or modified imported data from cross-sectional images to develop physical models specifically for teaching complex segments and branches of anatomy. 3D printing itself is easily accessible through online commercial services, and the models are made of polyamide or plaster. | Anatomical models of the liver, lungs, prostate, coronary arteries, and the Circle of Willis were created. These models have advantages that include customizable details, relative low cost, complete control of design focused on subsegments, potential for color coding, and the use of cross-sectional images combined with graphic design. | The radiologists have the opportunity to serve as leaders in medical education and clinical care with 3D printed models that provide beneficial interaction with patients, physicians, and learners in all specialties, proactively assuming the role of educator. Complex models can be developed to demonstrate normal anatomy or common pathology for medical education purposes. There is a need for randomized trials, which radiologists can design, to demonstrate the utility and effectiveness of 3D printed models for teaching simple and complex anatomy, simulating interventions, measuring patient satisfaction, and improving clinical care. | Simulator Configuration | In some cases, the organ had to be subdivided or manufactured in parts, so that the assembly and disassembly facilitate the illustration of the concepts and parts of the organ, including the use of marker paint or spray to further facilitate the didactics of the simulator. | Section the virtual model before proceeding to manufacture | Ease of didactics, according to the criteria of a radiologist or specialist. |
| GME1 | Biomodels for education and simulation | Biomodels | ME12 | Design and Manufacture with 3D printing, Anatomical models for teaching | They graphically designed 3D meshes or modified imported data from cross-sectional images to develop physical models specifically for teaching complex segments and branches of anatomy. 3D printing itself is easily accessible through online commercial services, and the models are made of polyamide or plaster. | Anatomical models of the liver, lungs, prostate, coronary arteries, and the Circle of Willis were created. These models have advantages that include customizable details, relative low cost, complete control of design focused on subsegments, potential for color coding, and the use of cross-sectional images combined with graphic design. | The radiologists have the opportunity to serve as leaders in medical education and clinical care with 3D printed models that provide beneficial interaction with patients, physicians, and learners in all specialties, proactively assuming the role of educator. Complex models can be developed to demonstrate normal anatomy or common pathology for medical education purposes. There is a need for randomized trials, which radiologists can design, to demonstrate the utility and effectiveness of 3D printed models for teaching simple and complex anatomy, simulating interventions, measuring patient satisfaction, and improving clinical care. | Simulator Price | It should be economical enough to compete with similar anatomical models. | Select scale according to search price formula and current model price. | Use of formula to choose scale (scale = (desired price / current price) ^ 1/3), The cost of each model depends on the size and amount of material used, with a total cost of each model ranging approximately between $40 and $100. |
| GME1 | Biomodels for education and simulation | Biomodels | ME12 | Design and Manufacture with 3D printing, Anatomical models for teaching | They graphically designed 3D meshes or modified imported data from cross-sectional images to develop physical models specifically for teaching complex segments and branches of anatomy. 3D printing itself is easily accessible through online commercial services, and the models are made of polyamide or plaster. | Anatomical models of the liver, lungs, prostate, coronary arteries, and the Circle of Willis were created. These models have advantages that include customizable details, relative low cost, complete control of design focused on subsegments, potential for color coding, and the use of cross-sectional images combined with graphic design. | The radiologists have the opportunity to serve as leaders in medical education and clinical care with 3D printed models that provide beneficial interaction with patients, physicians, and learners in all specialties, proactively assuming the role of educator. Complex models can be developed to demonstrate normal anatomy or common pathology for medical education purposes. There is a need for randomized trials, which radiologists can design, to demonstrate the utility and effectiveness of 3D printed models for teaching simple and complex anatomy, simulating interventions, measuring patient satisfaction, and improving clinical care. | structural integrity of the simulator | The minimum wall thickness must be large enough to ensure the structural integrity of the model | Select wall size at the discretion or suggestion of the manufacturer. | Select minimum wall thickness at the discretion of the manufacturer. |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Simulator material | The simulator material must resemble the real tissues that will be intervened | Vascular System and Aneurysm: Shore A 27 hardness photopolymer material (Objet500 Connex 3D printer), translucent whitish vascular models were immersed in a red dye bath. Skull: printed composite material (zPrinter 650). Brain: a silicone (Dragon Skin, Smooth-On Inc., Macungie PA) with a lower elastic modulus than the mold, then mixed according to the manufacturer's instructions with a pigment additive (Silc Pig, Smooth-On Inc., Macungie PA). A touch mutator (Slacker, Smooth-On Inc., Macungie PA) further reduced the elastic modulus. | Vascular System: manufacturer's technical specifications for material hardness Soft material that simulates soft tissues. Skull: Drilling tests are performed by specialists. Brain: Additives are added to lower the elastic modulus. |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Simulator Geometry | The geometry should be as close as possible to that of a real and average patient. | Aneurysm: From 9 sets of patient data from computed tomography angiography were imported to Mimics (Materialise, Leuven, Belgium), a medical reconstruction software package, the process of dividing an image into parts, was performed to isolate the geometries of aneurysmal and parental vessels, synthesized into a single computational model of the Circle of Willis, develops the hollow feature of the vessel core; Brain: the magnetic resonance imaging data set of a healthy patient was imported into Mimics and segmented, the resulting surface mesh was then imported into Geomagics, where partitioning facilitated the extraction of brain components to adjust or replace brain aneurysms without dismantling the entire simulation. Skull: A computed tomography data set, from a patient with normal cranial morphology, was imported into Mimics and segmented. | Simulate the real experience |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Material of Models for Mold | These materials will be used for the manufacture of the Mold. It does not require special load resistance qualities except for the curing temperatures during mold manufacturing, the most desirable quality is dimensional accuracy to replicate the patient's members. | Brain model: A Stratasys Dimension 1200es 3D printer (Eden Prairie, Minnesota, USA) was used to print the final computational models in acrylonitrile butadiene styrene plastic. The surface underwent partial chemical dissolution with a 90:10 volume solution of xylene and acetone to remove visible streaks created during the 3D printing process. | |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Geometry of Models for mold | It should be in the specific shape and size customized for the patient (bone tomography). | Brain model: The magnetic resonance imaging data of a healthy patient was imported into Mimics and segmented. The resulting surface mesh was then imported into Geomagics, where it was partitioned. The partitioning facilitated the operation of the simulator in extracting the brain, components to adjust or replace brain aneurysms without dismantling the entire setup. The mesh consists of 6 separable components: 1) the left frontal and parietal lobes, 2) the right frontal and parietal lobes, 3) the left temporal and occipital lobes, 4) the right occipital and temporal lobes, 5) the cerebellum, and 6) the brainstem (with a truncated portion of the optic nerves). | Simulate the real experience |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Geometry of the Mold | The geometry should be as close as possible to that of a real and average patient. | Brain Model: It was used to print the final computational models in acrylonitrile butadiene styrene plastic. The surface underwent partial chemical dissolution with a 90:10 volume solution of xylene and acetone to remove visible streaks created during the 3D printing process. A 2-part mold was created around each solid component of the brain using commercially available casting silicone (Mold Star [Smooth-On, Easton, Pennsylvania, United States]). These mold parts defined the negative shape of the intended brain. | Simulate the real experience |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Mold Material | The material must guarantee the geometry of the simulator and facilitate demolding. | A two-part mold was created around each solid component of the brain using a commercially available casting silicone (Mold Star [Smooth-On, Easton, Pennsylvania, United States]). These mold parts defined the negative shape of the intended brain. | Ease of manufacturing and physical integrity during successive demolding operations |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Simulator Configuration | The design must be modular. It must be subdivided to facilitate assembly and disassembly, the insertion of elements for academic study of different types of aneurysms, and maintenance by changing spare parts without implying the destruction of the entire simulator, and reducing maintenance costs. | Section the virtual model before proceeding to manufacture | Ease of teaching and maintenance |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Simulator Price | It should be economical enough during operation and maintenance. | Section the virtual model before proceeding to manufacture, the vascular model is modular. | A modular design was developed, following functional academic and maintenance cost criteria. Although the entire simulation costs less than $1000 for initial manufacturing, modular constructions allow for lower cost (less than $10 material cost) replacement of worn parts and enable the insertion of a specific aneurysm to fit specific teaching objectives. |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | structural integrity of the simulator | The size of smaller details is limited by the machine's limits, 1mm | It was decided not to manufacture details of vessels and minor nerves less than 1mm, as well as not to manufacture the arachnoid membrane that covers the brain. | According to the manufacturer's criteria and machine limitations. |
| GME1 | Biomodels for education and simulation | Surgical simulators | ME13 | The authors developed a manual, and a dimensionally accurate model for aneurysm clipping using patient-derived anatomical data and three-dimensional (3D) printing. The model design focused on reproducibility as well as adaptability to the patient's new geometry. | A modular, reproducible, and patient-derived medical simulation was developed for medical students to practice aneurysm clipping procedures. Various forms of 3D printing were used to develop an accurate geometry of the skull and vascular tree with 9 patient-derived aneurysms using 3D printing in conjunction with elastomers. Molding was utilized to achieve a patient-derived brain model with tactile properties not yet available in the market for 3D printing technology. An educational pilot study was conducted to measure the effectiveness of the simulation. | Through the innovative manufacturing process, a patient-derived simulator was developed for neurovascular surgical simulation. A qualitative follow-up study suggests potential for improving current educational programs; evaluations support the effectiveness of the simulator. | The proposed aneurysm clipping simulator has the potential to enhance learning experiences in the surgical environment. 3D printing and elastomeric molding can produce patient-derived models for learning in a dynamic environment that adds value to surgical training and preparation. | Functionality test | Professionals related must test the simulator and provide feedback with their opinion. | To qualitatively validate the simulation, 14 neurosurgery residents interacted with the simulation under the guidance of a neurosurgeon and provided qualitative feedback on its form and function. The evaluation covered the realism of the simulation when interacting with surgical tools (e.g., a bone drill) and medical devices (e.g., vascular clips). A simple survey assessing the clinical applicability, realism, and educational value of the simulation was completed by medical professionals. The survey consisted of 7 questions, and ratings were given on a 5-point Likert scale. | Fourteen residents of neurosurgery, with an average postgraduate year of 3.3 (i.e., range, 1-6 years), performed the Orbitozygomatic and Conventional Craniotomies for the first time on the skull model. Aneurysm clips were applied to occlude the aneurysms, including in the vascular model. The average response for all survey questions was greater than 4 (range 4.1-4.6) on the 5-point scale. The simulator is clinically applicable 4.4 (response range: 1 to 5, 1 strongly negative, 5 strongly positive), improved understanding of the aneurysm with respect to the parent artery 4.4, improved understanding of surgical vision 4.5, the application of the clip seemed realistic 4.1, bone drilling seemed realistic 4.1, the simulator was useful 4.6, and believed that surgical skills can improve with practice with the simulator 4.4. |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | Simulator material | The simulator material must resemble the real tissues that will be intervened | Synthetic gelatin as a tactically realistic material for casting, widely used as a simulating fabric, synthetic gelatin is transparent, storage-stable gelatin that is mechanically identical to classic organic 10% gelatin, a mixture of 1000 g of gelatin and 9000 ml of water. With a density of 1060 kg/m3, synthetic gelatin has a similar density to most soft biological tissues. With a rigidity of the order of 10 kPa, it is 5 orders of magnitude softer than common 3D printable materials and only one order of magnitude stiffer than brain tissue. The cost of the gelatin model material is $22, the gelatin can be reused by melting and molding, and creating the gelatin model requires 3 hours. | Soft material that simulates soft tissues, After probing a selection of suitable organic and synthetic materials regarding their structural integrity, rigidity, and cutting properties. |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | Geometry simulator | The geometry should be as close as possible to that of a real and average patient. | Magnetic resonance images of a healthy 25-year-old woman. FreeSurfer (an image analysis tool that is documented and freely available online) was used to calculate brain volume, surface area, cortical thickness, and gyrification indices, and to create stereolithography files of the left and right brain hemispheres. Creating the brain surface model is free of charge and takes approximately 4 hours, fully automated, on a standard desktop computer. | Simulate the real experience |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | Material of Models for Mold | These materials will be used for the manufacture of the Mold. It does not require special load resistance qualities except for the curing temperatures during mold manufacturing, the most desirable quality is dimensional precision to replicate the patient's members. | They were 3D printed on a FlashForge Creator Pro Dual Extrusion 3D printer (FlashForge, City of Industry, California, USA) using acrylonitrile butadiene styrene (ABS) thermoplastic with a filament diameter of 1.75 ± 0.05 mm and filament roundness of 0.07 mm (GizmoDorks, Temple City, California, USA). The current price of the 3D printer is $900, plastic filament costs approximately $4 per hemisphere, and the 3D printing process takes 10 hours, without supervision. | |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | Geometry of Models for mold | The implant and bone tissue must be of the specific shape and size customized for the patient (bone tomography) | Computed Tomography of Patient / Dicom Postprocessed in meshmixer, Solidwork, simplify3d | Simulate the real experience |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | Geometry of the Mold | The geometry should be as close as possible to that of a real and average patient. | It was printed on a 3D brain model that was used as a template in a molding process. | Simulate the real experience |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | Mold Material | The material must guarantee the geometry of the simulator and facilitate demolding. | To create a realistic and deformable tactile brain model, a 3D brain model was printed and used as a template in a molding process. Flexible molds were created using Rebound 25 (Smooth-On, Macungie, Pennsylvania, USA), a Shore 25 hardness silicone with a tensile strength of 700 kPa, along with Plasti-Paste II (Smooth-On), a mother mold to maintain structural integrity. The mixture was brushed onto the left and right hemispheres in 4 layers to create a strong and durable mold for casting. The cost of the silicone mold material is $20, and its creation takes approximately 7 hours. | Ease of manufacturing and physical integrity during successive demolding operations |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | Simulator Price | Prices should be considered at each stage of manufacturing, including prices. | Prices are accounted for at each stage of manufacturing, as well as the times. | Creating the brain surface model is free of charge and takes approximately 4 hours, completely automated, on a standard desktop computer. using acrylonitrile butadiene styrene (ABS) thermoplastic with a filament diameter of 1.75 ± 0.05 mm and filament roundness of 0.07 mm (GizmoDorks, Temple City, California, USA). The current price of the 3D printer is $900, plastic filament costs approximately $4 per hemisphere, and the 3D printing process takes 10 hours, unsupervised. The material cost for the silicone mold is $20, and its creation takes approximately 7 hours. The material cost for the gelatin model is $22, the gelatin can be reused through melting and molding, and the creation of the gelatin model requires 3 hours. |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | structural integrity of the simulator | The size of smaller details is limited by the machine's limits, 1mm | To characterize the mechanical properties of the gelatin model, 3 nanoindentation tests were performed on 5 mm thick brain slice models and compared to similar nanoindentation tests on sagittal slices of mammalian brains. To quantify potential mechanical degradation of the model, fresh brain slices were tested and compared to slices tested 3 months after fabrication. | In nanoindentation tests, the gelatin model conceptually exhibits characteristics similar to mammalian brain: it behaved like an ultra-soft and viscoelastic polymer. With a stiffness of E = 25.29+/-2.68 kPa, the cuts of the brain model (right) were less than an order of magnitude stiffer than mammalian brain slices (left) with a stiffness of E = 2.64+/-0.40 kPa. The shape of the indentation curve of the substitute material closely mimicked the rheology of mammalian brain tissue: both curves show a straight loading curve, a drop in force at constant deformation characteristic of viscous relaxation, a slightly concave upward discharge curve, and a negative force at the end of discharge characteristic of soft adhesion between the probe and the penetration tip. The mechanical properties of the brain model only moderately changed over time: with a stiffness of E = 27.64+/-0.37 kPa, the slices of new models were slightly stiffer than a 3-month-old model with a stiffness of E = 22.93+/-1.08 kPa but otherwise showed similar rheology. |
| GME1 | Biomodels for education and simulation | Biomodels | ME14 | In this technical note, the authors present a new technology for creating deformable and personalized models of the human brain. | The method combines 3D printing, molding, and casting to create a realistic physiological, anatomically based tactile model from magnetic resonance imaging images. Created from soft silicone, the model is easy to produce, cost-effective, durable, and several orders of magnitude softer than conventionally printed 3D models. The custom brain cost $50 USD and took 24 hours to manufacture. | In mechanical tests, the stiffness of the model (E=25.29+/-2.68 kPa) was 5 orders of magnitude softer than common 3D printed materials, and less than one order of magnitude stiffer than mammalian brain tissue (E=2.64+/-0.40 kPa). In a multicenter surgical survey, the size of the model (100.00%), visual appearance (83.33%), and surgical anatomy (81.25%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95.00%). | With minor refinements, Deformable custom brain models created through 3D printing enhance surgical training and preoperative planning with the ultimate goal of providing high precision, customization, and accuracy. | Functionality test | Professionals related must test the simulator and provide feedback with their opinion. | To characterize the functional features of the model, the deformable gelatin model was evaluated by 10 neurosurgeons and residents from King's College London, University of Oxford, and Stanford University. The surgeons were asked to evaluate the model and its usefulness as a training and neurosurgical planning tool. | Feedback surveys from 10 neurosurgeons and residents revealed overall satisfaction with the model and a wide range of potential uses. Surgical satisfaction with the model's rigidity (43.75%), cutting properties (43.75%), and haptic feedback (56.25%) could still be improved, while anatomy (81.25%), visual appearance (83.33%), and model size (100%) were perceived as very realistic. The model was perceived as very useful for patient illustration (85.00%), teaching (94.44%), learning (100.00%), surgical training (95.00%), and preoperative planning (95%). All surgeons responded that they would actively use the model for one or more of these purposes (100%). The survey suggests that the model will be highly useful for training and planning surgical procedures, including, among others, tumor removal (70%), aneurysm treatment (70%), fissure dissection (20%), and electrode placement (10%). The model could be anatomically improved by including vasculature (60%), ventricles (40%), individual tumors (30%), fissures (20%), brainstem (10%), arachnoid (10%), and cerebrospinal fluid (10%). By using different colors for different regions of the brain, the model could also be visually enhanced (20%). The overall response was very positive based on the justification that only a few training and surgical planning tools are currently available, and none of them have realistic tactile touch and mechanical properties. |
| GME2 | Surgical planning biomodels | Biomodels | ME18 | Explore the effect of 3D printing-assisted cognitive fusion on improving the positive rate of prostate biopsy. | From August to December 2014, 16 patients with suspected prostate lesions were detected by multiparametric magnetic resonance imaging (MRI) and included in the study. Prostate biopsy was performed using 3D models of prostate reconstruction, computer-simulated biopsy, 3D printing, and cognitive fusion biopsy. All patients had received multiparametric 3.0 T magnetic resonance imaging before the biopsy. The MRI DICOM files were imported using medical imaging software for 3D reconstruction modeling to generate a printable stl file for 3D printing, using transparent resin as the raw material. A biopsy of 2 to 3 cores was performed with suspected lesions detected on magnetic resonance imaging. | For the 16 patients in the present study, 3D modeling with fusion-based cognitive targeting biopsy was successful. For a single patient, 1-2 lesions (average: 1.1 lesions) were discovered, followed by 2-6 cores (average: 2.4 cores) added as target biopsy. Systematic biopsies represented a total of 192 cores, with a positive rate of 22.4%; targeted biopsies represented a total of 39 cores, with a positive rate of 46.2%. Among these cases, 10 patients (62.5%) were diagnosed with prostate adenocarcinoma, in which seven were discovered by systematic and targeted biopsy, one was diagnosed by systematic biopsy only, and two were diagnosed by targeted biopsy only. For systematic biopsy, the Gleason score ranged from 6 to 8 (average: 7), while for targeted biopsy, it ranged from 6 to 9 (average: 7.67). Among the seven patients who were diagnosed by systematic and targeted biopsy, three (42.8%) were reported with a higher Gleason score in the targeted therapy than in the systematic biopsy. | The 3D printing technique was applied to aid cognitive fusion in the early diagnosis of prostate cancer, which significantly improved the positive biopsy rate and avoided misdiagnosis of high-risk prostate cancer. This technique proves to be easy and simple. The increased effort in targeted biopsy does not increase the incidence of complications. Its application and popularization will surely benefit more patients on a larger scale in the future. | Simulator material | The material application can intuitively show the location, size, and morphology of the tumor. | Transparent resin material used for 3D printing model, tumor's spatial structure. | Before the biopsy, the operator can observe a 3D model of the tumor from multiple angles, thus evaluating the possibility of sampling by systematic biopsy or cognitive fusion. |
| GME2 | Surgical planning biomodels | Biomodels | ME18 | Explore the effect of 3D printing-assisted cognitive fusion on improving the positive rate of prostate biopsy. | From August to December 2014, 16 patients with suspected prostate lesions were detected by multiparametric magnetic resonance imaging (MRI) and included in the study. Prostate biopsy was performed using 3D models of prostate reconstruction, computer-simulated biopsy, 3D printing, and cognitive fusion biopsy. All patients had received multiparametric 3.0 T magnetic resonance imaging before the biopsy. The MRI DICOM files were imported using medical imaging software for 3D reconstruction modeling to generate a printable stl file for 3D printing, using transparent resin as the raw material. A biopsy of 2 to 3 cores was performed with suspected lesions detected on magnetic resonance imaging. | For the 16 patients in the present study, 3D modeling with fusion-based cognitive targeting biopsy was successful. For a single patient, 1-2 lesions (average: 1.1 lesions) were discovered, followed by 2-6 cores (average: 2.4 cores) added as target biopsy. Systematic biopsies represented a total of 192 cores, with a positive rate of 22.4%; targeted biopsies represented a total of 39 cores, with a positive rate of 46.2%. Among these cases, 10 patients (62.5%) were diagnosed with prostate adenocarcinoma, in which seven were discovered by systematic and targeted biopsy, one was diagnosed by systematic biopsy only, and two were diagnosed by targeted biopsy only. For systematic biopsy, the Gleason score ranged from 6 to 8 (average: 7), while for targeted biopsy, it ranged from 6 to 9 (average: 7.67). Among the seven patients who were diagnosed by systematic and targeted biopsy, three (42.8%) were reported with a higher Gleason score in the targeted therapy than in the systematic biopsy. | The 3D printing technique was applied to aid cognitive fusion in the early diagnosis of prostate cancer, which significantly improved the positive biopsy rate and avoided misdiagnosis of high-risk prostate cancer. This technique proves to be easy and simple. The increased effort in targeted biopsy does not increase the incidence of complications. Its application and popularization will surely benefit more patients on a larger scale in the future. | Simulator Geometry | The translated value of the provided data La geometría debe ser la de del paciente especifico in English is The geometry should be that of the specific patient. | All patients had received a multiparametric 3.0 T magnetic resonance imaging (Siemens Magnetom Skyra, Germany) before the biopsy. The scanning sequence included T1-weighted, T2-weighted, DCE, and DWI. | The prostate is a soft tissue organ, so processing MRI data images is more difficult than that of bones, teeth, and other tissues. In the data modeling phase, a lot of recognition of pelvic anatomy is required, with the help of imaging professionals. Therefore, based on various multiparametric magnetic resonance fusions, targeted biopsy cannot completely replace systematic biopsy. The development of targeted biopsy is based on more sensitive advances and specific imaging technologies. |
| GME2 | Surgical planning biomodels | Biomodels | ME18 | Explore the effect of 3D printing-assisted cognitive fusion on improving the positive rate of prostate biopsy. | From August to December 2014, 16 patients with suspected prostate lesions were detected by multiparametric magnetic resonance imaging (MRI) and included in the study. Prostate biopsy was performed using 3D models of prostate reconstruction, computer-simulated biopsy, 3D printing, and cognitive fusion biopsy. All patients had received multiparametric 3.0 T magnetic resonance imaging before the biopsy. The MRI DICOM files were imported using medical imaging software for 3D reconstruction modeling to generate a printable stl file for 3D printing, using transparent resin as the raw material. A biopsy of 2 to 3 cores was performed with suspected lesions detected on magnetic resonance imaging. | For the 16 patients in the present study, 3D modeling with fusion-based cognitive targeting biopsy was successful. For a single patient, 1-2 lesions (average: 1.1 lesions) were discovered, followed by 2-6 cores (average: 2.4 cores) added as target biopsy. Systematic biopsies represented a total of 192 cores, with a positive rate of 22.4%; targeted biopsies represented a total of 39 cores, with a positive rate of 46.2%. Among these cases, 10 patients (62.5%) were diagnosed with prostate adenocarcinoma, in which seven were discovered by systematic and targeted biopsy, one was diagnosed by systematic biopsy only, and two were diagnosed by targeted biopsy only. For systematic biopsy, the Gleason score ranged from 6 to 8 (average: 7), while for targeted biopsy, it ranged from 6 to 9 (average: 7.67). Among the seven patients who were diagnosed by systematic and targeted biopsy, three (42.8%) were reported with a higher Gleason score in the targeted therapy than in the systematic biopsy. | The 3D printing technique was applied to aid cognitive fusion in the early diagnosis of prostate cancer, which significantly improved the positive biopsy rate and avoided misdiagnosis of high-risk prostate cancer. This technique proves to be easy and simple. The increased effort in targeted biopsy does not increase the incidence of complications. Its application and popularization will surely benefit more patients on a larger scale in the future. | Aesthetics | The material application can intuitively show the location, size, and morphology of the tumor. | The files .stl were printed with a thickness of 0.1 mm. The printing material was transparent resin, and inside the suspicious defect was colored. | Before the biopsy, the operator can observe a 3D model of the tumor from multiple angles, thus evaluating the possibility of sampling by systematic biopsy or cognitive fusion. |
| GME2 | Surgical planning biomodels | Biomodels | ME18 | Explore the effect of 3D printing-assisted cognitive fusion on improving the positive rate of prostate biopsy. | From August to December 2014, 16 patients with suspected prostate lesions were detected by multiparametric magnetic resonance imaging (MRI) and included in the study. Prostate biopsy was performed using 3D models of prostate reconstruction, computer-simulated biopsy, 3D printing, and cognitive fusion biopsy. All patients had received multiparametric 3.0 T magnetic resonance imaging before the biopsy. The MRI DICOM files were imported using medical imaging software for 3D reconstruction modeling to generate a printable stl file for 3D printing, using transparent resin as the raw material. A biopsy of 2 to 3 cores was performed with suspected lesions detected on magnetic resonance imaging. | For the 16 patients in the present study, 3D modeling with fusion-based cognitive targeting biopsy was successful. For a single patient, 1-2 lesions (average: 1.1 lesions) were discovered, followed by 2-6 cores (average: 2.4 cores) added as target biopsy. Systematic biopsies represented a total of 192 cores, with a positive rate of 22.4%; targeted biopsies represented a total of 39 cores, with a positive rate of 46.2%. Among these cases, 10 patients (62.5%) were diagnosed with prostate adenocarcinoma, in which seven were discovered by systematic and targeted biopsy, one was diagnosed by systematic biopsy only, and two were diagnosed by targeted biopsy only. For systematic biopsy, the Gleason score ranged from 6 to 8 (average: 7), while for targeted biopsy, it ranged from 6 to 9 (average: 7.67). Among the seven patients who were diagnosed by systematic and targeted biopsy, three (42.8%) were reported with a higher Gleason score in the targeted therapy than in the systematic biopsy. | The 3D printing technique was applied to aid cognitive fusion in the early diagnosis of prostate cancer, which significantly improved the positive biopsy rate and avoided misdiagnosis of high-risk prostate cancer. This technique proves to be easy and simple. The increased effort in targeted biopsy does not increase the incidence of complications. Its application and popularization will surely benefit more patients on a larger scale in the future. | Simulator Configuration | The print should include the external geometry of the organ, but also within this geometry of the suspicious area. | Images of prostate and tumor were introduced for the reconstruction of the 3D model and smoothly processed to generate printable files in .stl format. Through the SLA 3D printer, RS-450, the printing material was transparent resin, and inside the color defect. | Before the biopsy, the operator can observe a 3D model of the tumor through the organ model from multiple angles, thus evaluating the possibility of sampling by systematic biopsy or cognitive fusion. |
| GME2 | Surgical planning biomodels | Biomodels | ME18 | Explore the effect of 3D printing-assisted cognitive fusion on improving the positive rate of prostate biopsy. | From August to December 2014, 16 patients with suspected prostate lesions were detected by multiparametric magnetic resonance imaging (MRI) and included in the study. Prostate biopsy was performed using 3D models of prostate reconstruction, computer-simulated biopsy, 3D printing, and cognitive fusion biopsy. All patients had received multiparametric 3.0 T magnetic resonance imaging before the biopsy. The MRI DICOM files were imported using medical imaging software for 3D reconstruction modeling to generate a printable stl file for 3D printing, using transparent resin as the raw material. A biopsy of 2 to 3 cores was performed with suspected lesions detected on magnetic resonance imaging. | For the 16 patients in the present study, 3D modeling with fusion-based cognitive targeting biopsy was successful. For a single patient, 1-2 lesions (average: 1.1 lesions) were discovered, followed by 2-6 cores (average: 2.4 cores) added as target biopsy. Systematic biopsies represented a total of 192 cores, with a positive rate of 22.4%; targeted biopsies represented a total of 39 cores, with a positive rate of 46.2%. Among these cases, 10 patients (62.5%) were diagnosed with prostate adenocarcinoma, in which seven were discovered by systematic and targeted biopsy, one was diagnosed by systematic biopsy only, and two were diagnosed by targeted biopsy only. For systematic biopsy, the Gleason score ranged from 6 to 8 (average: 7), while for targeted biopsy, it ranged from 6 to 9 (average: 7.67). Among the seven patients who were diagnosed by systematic and targeted biopsy, three (42.8%) were reported with a higher Gleason score in the targeted therapy than in the systematic biopsy. | The 3D printing technique was applied to aid cognitive fusion in the early diagnosis of prostate cancer, which significantly improved the positive biopsy rate and avoided misdiagnosis of high-risk prostate cancer. This technique proves to be easy and simple. The increased effort in targeted biopsy does not increase the incidence of complications. Its application and popularization will surely benefit more patients on a larger scale in the future. | Functionality test | The effectiveness of the 3D printing-assisted fusion technique should be compared with the systematic or traditional technique. | Sixteen patients with suspected prostatic lesions were detected by multiparametric magnetic resonance imaging (MRI), who were included in the study. Prostate biopsy was performed using 3D models of prostate reconstruction, computer-simulated biopsy, 3D printing, and cognitive fusion biopsy. All patients had received multiparametric 3.0 T magnetic resonance imaging before the biopsy. The operator can observe the 3D model of the tumor through the organ model from multiple angles, thus evaluating the possibility of sampling by systematic biopsy or cognitive fusion. | For a single patient, 1-2 lesions were discovered, followed by 2-6 cores aggregated as target biopsy. Systematic biopsies represented a total of 192 cores, with a positive rate of 22.4%; Directed biopsies represented a total of 39 cores, with a positive rate of 46.2%. Among these cases, 10 patients (62.5%) were diagnosed with prostate adenocarcinoma, in which seven were discovered by systematic and directed biopsy, one was diagnosed by systematic biopsy only, and two were diagnosed by directed biopsy only. For systematic biopsy, the Gleason score ranged from 6 to 8 (average: 7), while for directed biopsy, it ranged from 6 to 9 (average: 7.67). Among the seven patients who were diagnosed by systematic and directed biopsy, three (42.8%) were reported with a higher Gleason score in the directed therapy than in the systematic biopsy. |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | Ease of design | Design software plays a significant role in product design and development for creating design by requirement as limitation requires high design skill. Design is also created from CT, MRI, and 3D scanners, which helps generate and produce in less time. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | Material | A wide variety of materials such as plastic, metals, composites, wood, and alloyed materials are used, with the limitation that not all AM processes handle all materials. Changing materials creates models with the required strength. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | Color in ingles language is Color. | In cardiology, one can undertake a full-color study of the 3D heart model before starting the actual surgery, although some AM technologies are monochrome. Through this, everything can be seen, the veins, the flow, and blockage in the heart. 3D inkjet printers can easily print a full-color model and fulfill this requirement. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | Efficiency | AM improves aspects of sustainability and resource efficiency, although the quality of the raw material affects the performance of the entire process. It improves material use efficiency, manufacturing process, and design. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | speed | The translation of the provided data into English is: The printing speed changes depending on the manufacturing orientation, although in general the limitation is in mass production, but it is fine for custom manufacturing. Cardiologists need to have the printed model before surgery. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | Model Dimensions | Print any shape and size of product by implant geometry, any sophisticated shape can be manufactured efficiently. The limitation is the volume of the printer. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | cost | For implants or medical models, AM successfully produces at low cost. The manufacturing of medical implants is easier and more personalized compared to machining. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | Precision | Improve accuracy through changes in layer height and resolution in the digital file. Changing raw material specifications affects model accuracy. Sometimes the raw material is not manufactured precisely. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | Easy to Use | The technology comfortably captures the medical image and converts it into a 3D model. The model produced by AM technology is easy to use because it does not require tools or accessories. However, it does require personnel to be trained in this technology. | - | - |
| GME2 | Surgical planning biomodels | Biomodels | ME31 | This review describes and illustrates the application, development, and limitations associated with AM in the field of cardiology by studying articles in AM medicine/cardiology. | Relevant articles until August 2018 were identified through Scopus and examined according to their strengths, benefits, limitations, contributions, and future potential for additive manufacturing. With the help of existing literature and bibliographic analysis, different applications in cardiology are being investigated. | I apologize, but I am not able to provide translation services at the moment. My capabilities are limited to providing information and answering questions. | AM has the potential to be an immense aid to cardiologists and cardiac surgery in interventions and surgical planning, monitoring and analysis. AM creates a 3D model of a specific patient's heart in less time and cost. This technology is used to create and analyze 3D models before starting surgery on the patient. This can improve treatment for the patient, saving lives. The article summarizes the application of AM in cardiology. Printed models reduce risks, improve the quality of diagnosis and preoperative planning, as well as team communication. In cardiology, patient heart data varies from patient to patient, so AM efficiently produces 3D models by converting pre-designed virtual models into tangible objects. Companies are exploring additive manufacturing for medical commercial applications. | Simulation before and after medication | AM can attend advanced simulations in cardiology for healthcare solutions. Simulation of trained doctors and virtual reality predict changes after surgery and medication. This presents a critical and innovative way of communicating and interacting. With the limitation that it is not always reliable. | - | - |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Function | It must be coupled with the patient's stump and with the prosthetic knee, it transfers the patient's weight from the top | The patient's stump is scanned in 3D, the size is modified in SolidWorks, support fins are added, and a hole is added for the stump suction valve, and a coupling for the knee is added. | The scanning and modification process in SolidWorks for direct 3D printing manufacturing reduces the number of steps and direct contact with the orthopedic technician. The hole for the valve, knee coupling, and size change are recommendations based on orthopedic empirical rule and other socket models. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Aesthetic/ergonomics | The surface must be smooth both on the inside for contact with the patient, and on the outside for aesthetic appearance. | Proceed to sand with sandpaper and choose a white color for the ABS. | The process of sanding reduces the roughness associated with the layer manufacturing step defect. The white color makes it easier to achieve a smooth finish, the color hides the sanding scratches that would be noticeable for other colors. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb are designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Customization | The socket is the result of the shape and size of the patient's stump, with a reduction in size based on an orthopedic empirical rule, which takes into account the consistency of the stump, that is, the stump is not a rigid tissue and will contract due to the weight of the patient, and compression against the socket. | Scanning with a 3D scanner, kinet for Windows, is modified in Solidworks. | It is scanned with a 3D scanner, kinet for Windows, from the mesh file, a parametric solid/surface is generated in SolidWorks, a file that is easier to modify accurately, and the size of the socket is reduced according to the orthopedic empirical rule that considers the consistency of the stump. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb are designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Dimensions | The socket is the result of the shape and size of the patient's stump, with a reduction in size based on an orthopedic empirical rule, which takes into account the consistency of the stump, that is, the stump is not a rigid tissue and will contract due to the patient's weight and compression against the socket. The size depends on the size of the stump, if it exceeds the size of the printer, the socket must be manufactured in parts. | Scanning with a 3D scanner, kinet for Windows, modifying it in Solidwork, in case it exceeds the printer volume, it is divided and manufactured in parts. | Scanning is done with a 3D scanner, kinet for Windows, from the mesh file, a solid/surface is generated for solid in SolidWorks, a file that is easier to modify accurately, and the size of the socket is reduced according to the orthopedic empirical rule that considers the consistency of the stump. In case the printer volume is exceeded, it is divided and manufactured in parts. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Forces | The socket must support the weight of the patient, so that the load is concentrated at the top of the socket and not at the base. Similarly, the material and thickness must guarantee the physical integrity of the piece. | An fin or protuberance is included in the socket, and ABS material manufactured with FFF with sufficient thickness (3mm-4mm) is chosen. | Once the socket is reduced to the size of the patient, fins are included with SolidWorks to support the load on the top of the socket, in accordance with orthopedic empirical rule. The thickness of the socket is verified with finite element simulation. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Materials | The material must withstand the compressive load of the patient's weight, and it must have a smooth surface for aesthetics and functionality. | ABS manufactured by FFF, in White. | ABS is chosen for its wear resistance and the possibility of being sanded to improve its appearance. White color is chosen for its aesthetics and the possibility of being sanded to conceal scratch marks. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Manufacturing and Assembly | Minimize direct contact of personnel. The size or socket size of the printer limits the number of parts per assembly, as well as the number of printers to meet demand. The demand for prostheses in the country was estimated at 1000 units per year. | For direct 3D printing, contact is necessary and limited to sanding and assembly processes only. Depending on the size and volume of the printer, the socket is manufactured in multiple parts or as a single piece. Similarly, the monthly/annual production will determine the number of printers required to meet the demand. In the case of 3D printing, approximately 10 printers with a volume of 25x25x25cm would be required. | 3D printing is ideal, and only requires direct contact during the sanding and assembly stage. The size and production of the socket will control the necessary sizes of printers and their number in the company. In the case of 3D printing, about 10 printers with a volume of 25x25x25cm would be required. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb are designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | assembly | The socket must fit with the patient's stump, and with the prosthetic knee. | A liner is chosen for the patient, and a suction valve is used to attach the stump to the socket, in the socket it implies making a hole for the valve, apart from the coupling with the knee. | The hole for the valve, knee coupling are recommendations based on orthopedic empirical rule and other socket models. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Useful Life and Maintenance | Doctors recommend changing prostheses every 3 years, the international ISO standard 10328:2006 specifies the life of prostheses according to load cycling or number of steps (2 million cycles) and maintenance involves adjustments in screws and angles in pyramids. | Expected lifespan ranges from 1 year for printed materials to 3 years for conventional materials. | Printed materials have lower fatigue and impact resistance than conventional materials. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Costs and Deadlines | The cost of selling similar products is over 1000 USD (complete prosthesis), the demand for prostheses in the country was estimated at 1000 units per year. | The case of socket prints directly it is possible to reduce the market sale prices to a fraction with respect to conventional costs, combining 3D scanning and file modification in Solidworks, to be more competitive, the process must be automated, the current process takes a long time in the file modification stage. | Through trial and error, the 3D scanning processes can be made more efficient with a rotating platform (3 minutes), but the conversion process from scanning file to solid/parasolid surface requires better computational resources, and the conversion process needs to be automated to be more competitive. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Security | The translated value of the provided data Los materiales no deben reaccionar con el cuerpo humano in English is Materials should not react with the human body. | ABS is used in applications for children's toys. | ABS is used in Lego pieces and toys for children. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Ergonomics | The socket will be in direct contact with the patient's stump, poor ergonomics and friction can generate health problems such as ulcerations and infections. | Scanning is done with a 3D scanner, kinet for Windows, it is modified in Solidworks. The interior is then polished with sandpaper and ABS is chosen. A liner is chosen for the patient, and a suction valve is used to attach the stump to the socket. | Scanning is done with a 3D scanner, kinet for Windows, from the mesh file, a solid/surface is generated for solid in SolidWorks, a file that is easier to modify accurately. The size of the socket is reduced according to the orthopedic empirical rule that considers the consistency of the stump. The sanding process reduces the roughness associated with the layer manufacturing defect, ABS is an ideal material that can be sanded. The liner and the hole for the valve are recommendations based on the orthopedic empirical rule and other socket models. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME77 | * Design fully customized and functional upper limb prosthetics for people with low resources disabilities. * Manufacture upper limb prosthetics using 3D printing. * Design fully customized and functional lower limb prosthetics for people with low resources disabilities. * Manufacture lower limb prosthetics using 3D printing. | • Start meeting: a meeting will be held between professionals, CE CAMILO officials, and company engineers to define design requirements and patient types. • Patient interviews: interviews and studies are conducted with a disabled patient of the upper limb and another with a disabled patient of the lower limb with low resources, in order to gather information for the development of personalized prostheses for them. • Socket development: the socket is designed and constructed using the 3D scanning and printing process; the socket is also constructed using the molding process and the results are compared. • Development and construction of a prototype: a prototype of an upper limb prosthesis and another for a lower limb is designed and constructed using 3D printing. • Patient testing: patient tests are conducted using the prosthesis prototype and information is obtained for redesign and improvement of product functionality. • Prototype redesign: with the information found in the tests, a biomechanical study is conducted and the initial prototype is redesigned. • Construction of final prosthesis: a fully functional upper limb prosthesis and a lower limb prosthesis are constructed using 3D printing for low-income people with disabilities. • Prosthesis evaluation: a one-month period (approx.) where the user will evaluate the final prosthesis in their daily life and in control tests at the CE CAMILO laboratory. | An FFF 3D printed test socket was developed with ABS material to test the printed prototypes developed during the project. The polycentric knee prototypes with two axes (4-bar mechanism), the sach-type foot, and the dynamic foot were modeled and mechanically and dynamically analyzed for ABS material using the finite element method, verifying the structural integrity of the elements, the correct dynamic behavior, and the deformation of the dynamic foot to ensure damping of the walking impact. The prototypes were assembled with standardized elements and non-standardized couplings, and walking tests were performed on a horizontal and inclined plane with a person weighing 80 to 90 kg. A speed and acceleration analysis was performed using a free program. The test was successful dynamically, and the prototypes exhibited sufficient resistance during the test. | 3D printed prosthetic elements (FFF) were developed with ABS material. Their mechanical and dynamic behavior was modeled using finite elements, and their behavior was consistent during gait tests with simulation, demonstrating the feasibility of developing prostheses for testing and training patients at much lower costs compared to conventional prostheses. | Legal Aspects | Requires INVIMA Registration for commercialization, as well as a system implemented in a company with good manufacturing practices, a gait laboratory, and an orthopedic technician. | Apart from the investment for invima registration, a good manufacturing practice system, a walking laboratory, and an orthopedic technician are required to meet invima requirements. | It was investigated and advised with INVIMA, orthopedic technician, and specified what was required for the sale of socket and prosthetic elements. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME78 | Through tension tests, the modulus of elasticity, yield strength, and tensile strength in different directions of the printed ABS material (FFF) are evaluated. The elastic constants are determined and the influence of the filling percentage on the mechanical strength of the material is evaluated. Elastic finite element modeling of a prosthetic foot subjected to bending. Failure modeling of the prosthetic foot using laminated composite material failure theory. Perform tests on the prosthetic foot to validate the models. | A design of experiments was carried out in order to characterize the properties of the material as a function of the direction and filling percentage. The experiment was conducted in two stages. In the first stage, experiment A, the filling direction was varied and the filling percentage was fixed at 100%. In the second stage, experiment B, the filling percentage was fixed at 33% and the direction was varied. The response variables are: modulus of elasticity, yield strength, and tensile strength. The experimental factors are: printing direction and filling percentage. The material is modeled as linear elastic and transversely isotropic. The elastic constants were used to model the elastic behavior of a prosthetic foot using finite element analysis with the SolidWorks/Abaqus program, and failure models for laminated composite materials were used to predict the mechanical failure of the prosthetic foot. Finally, the modeling of the prosthetic foot is corroborated in a bending experiment at the tip of the foot with the ankle fixed, using a universal testing machine and a mechanical press. | It is observed that the mechanical properties do not vary significantly in the direction of the printing layer and vary significantly in the perpendicular direction of the same, which allows modeling the material as linear elastic and transversely isotropic. The filling percentage significantly influences the mechanical properties of the material, it is observed that there is a linear variation of the properties with respect to the filling percentage. The actual elastic behavior of the foot corresponded to what was predicted by the linear elastic and transversely isotropic model and the finite element simulation. The failure did not occur as predicted by the Tsai Hill/Tsai Wu mechanical failure model for laminated composite materials. | The experimentally characterized the mechanical properties of ABS in the 3D printing process, a transversely isotropic behavior was found, the mechanical properties are significantly different in the layer plane and in the direction perpendicular to it. The elastic constants of the material are: E1=1.700GPa, E3=1.230GPa, v12=0.24, v13=0.24 and G13=0.728MPa. The influence of the filling percentage was evaluated and it was found that the material complies with the law of mixtures of continuous media. The experimental results on the prosthetic foot were consistent with the theoretical modeling for elastic behavior but not for mechanical failure. | see ME82 | see ME82 | see ME82 | see ME82 |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics. *Design, construction of customized prototype, and testing: and information is obtained. *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for the donation of prostheses. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Function | It must be able to perform a strong grip, fine grip, and typing (electromechanical), retract easily, must be able to change and fix the orientation of the wrist according to the required work, must be lightweight and require minimal effort to avoid injuring back muscles or the stump muscle (electromechanical). | Mechanics: an open model is chosen on the internet flexihand offered by international foundations (https://enablingthefuture.org/upper-limb-prosthetics/the-flexy-hand/) and available on thingiverse (https://www.thingiverse.com/thing:380665), and it is modified to meet functional requirements. Electromechanics: the flexihand is modified and designed to operate with a system of servo motors and pulleys, controlled by an Arduino board, and activated by myoelectric signals from the muscle above the stump. The change of functions is based on the Michelangelo myoelectric prosthesis (https://www.ottobock.com.co/prosthetics/upper-limb/solution-overview/axon-bus-prosthetic-system-with-michelangelo-hand/) | Different open models were downloaded from the internet and offered by foundations or Thingiverse. Through a trial and error process, models were discarded, selecting the flexihand model, which was perfected through a trial and error method to meet the requirements. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics *Design, construction of customized prototype, and testing: and information is obtained *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for the donation of prostheses. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Dimensions | Mechanics: According to the patient's size. Electromechanics: With enough internal space to accommodate the servo motors, control board, and power supply. | It was designed with a similar size to that of the patient and was manufactured. | It was designed with a similar size to that of the patient and was manufactured. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics *Design, construction of customized prototype, and testing: and information is obtained *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for prosthesis donation. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Movements | Hold tight, hold fine, Fingering (Electromechanical), Wrist rotation. | Electromechanics: An existing design with flexible joints, operated by strings and levers and a pivot operated by a pivot pin is chosen, in a housing and rigid fingers. Mechanics: Additionally, for the wrist, a spring and ball system is designed to fix and rotate. | By trial and error, the existing system is modified and improved, and a new wrist rotation system is designed. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics. *Design, construction of customized prototype, and testing: and information is obtained. *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for prosthesis donation. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Forces | The force to be developed by the prosthesis must be sufficient to hold objects such as a glass of water (<1kg), or a water bottle (1kg to 2kg), or a coin (fine motor skills). The friction forces must be high enough to achieve reliable grip. | *Electromechanics: Servomotors are chosen powerful enough to develop the necessary static loads for gripping. *Mechanics: The longest length of the prosthesis, and a system of ropes and pivots like a conventional prosthesis (cable) and screws to control the tightening or tension in the ropes, develops sufficient force and moment to meet requirements. The use of adhesive and higher friction textures (adapted gloves) improves the grip function with small objects (fine motor skills). | By trial and error, the existing system is modified and improved. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics *Design, construction of customized prototype, and testing: and information is obtained *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for the donation of prostheses. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Materials | The materials must allow for finger retraction, but the textures must be rigid and facilitate strong and fine grip functions. The materials of the strings must be rigid and non-stretchable, but resistant to wear and able to withstand loads without breaking. The socket or coupling with the patient's stump should facilitate adjustments with the stump by thermoforming. | To achieve finger retraction, polyurethane joints are used between the fingers for a strong and precise grip. ABS rigid material is used for the fingers and the rest of the prosthetic body casing to enhance grip. Neoprene adapted gloves are also used to increase friction. The selected strings are 4-strand braided fishing line made of Polyethylene (PE), with a diameter of number 8, capable of supporting a load of 100 Lb. In the case of electromechanical prosthetics, the socket is conventionally manufactured using conventional materials. In the case of electromechanical prosthetics, scaling the print to fit measurements is an option, and/or final adjustments can be made using thermoforming on ABS coupling, but preferably on PLA. | By trial and error, the existing system is modified and improved. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics *Design, construction of customized prototype, and testing: and information is obtained *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for the donation of prostheses. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Manufacturing and Assembly | The demand for upper limb prostheses in the country is not demanding, and represents a small fraction (1% to a maximum of 25%) of the demand for lower limb prostheses. | The demand for prostheses in the country is much lower in units per year than required for lower prostheses. In the case of 3D printing, a small fraction of the 10 printers (1 to 2 printers) would be required for the manufacture of lower limb prostheses. | The estimated number of prostheses was determined based on market studies in orthopedic laboratories (potential clients) by interviewing technical managers, orthopedic technologists, physiatrists, and using information from population censuses to extrapolate local figures to the rest of the country. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics *Design, construction of customized prototype, and testing: and information is obtained *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for prosthesis donation. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | assembly | Assembly ease, maximum assembly time 10 min. The time for adjustments due to thermoforming on the stub and tension adjustments on the strings is much longer (20 to 30 minutes). | assembly time is selected in 10 minutes. The times for thermoforming adjustments and string tension adjustments are much longer (20 to 30 minutes) | The assembly time is based on the experience of the project developers and orthopedic technicians. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics. *Design, construction of customized prototype, and testing: and information is obtained. *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for prosthesis donation. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Useful Life and Maintenance | Expected A prolonged life of 1 to 3 years for the prosthesis is expected. | Expected lifespan of 1 to 3 years based on low loads to be supported and material resistance. Periodic replacement of spare parts such as flexible joints and ropes, and periodic adjustments of coupling or trunnion, or ropes. | Printed materials have lower fatigue and impact resistance than conventional materials, but the loads are very low compared to the experience of lower prostheses. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics *Design, construction of customized prototype, and testing: and information is obtained *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for the donation of prostheses. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Costs and Deadlines | Electromechanics: Economy, Cost of prosthetic element, Sale cost 1000 to 2000 USD. Mechanics: Economy, Cost of prosthetic element, Maximum manufacturing cost of the element 1'000.000 COP or 250 USD. | Electromechanics: Maximum selling price 1000 to 2000 USD. Mechanics: 1'000.000 COP or 250 USD. | The market prices are studied and the lowest price is chosen as the upper limit. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics *Design, construction of customized prototype, and testing: and information is obtained *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for the donation of prostheses. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Ergonomics | Sizes are managed by patient sizes and custom socket manufacturing. | It was designed with a size similar to that of the patient and manufactured. The fit with respect to the socket is achieved through custom socket manufacturing (conventional method for electromechanical prostheses) or through scaled printing and thermoforming adjustment (mechanical). | It was designed with a size similar to that of the patient and manufactured. A socket is manufactured conventionally for an electromechanical prosthesis, and by trial and error, a mechanical prosthesis coupling is manufactured and adjusted on a scale by thermoforming. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior prosthesis, inferior prosthesis | ME79 | Design electromechanical prostheses for upper and lower limbs, fully customized and functional, for people with disabilities from low-income backgrounds and victims of landmines. Manufacture upper and lower limb prostheses using 3D printing. Obtain international permits and certifications for the manufacturing and commercialization of prosthetic elements for lower limb prostheses. Donate prostheses and prosthetic elements to people with disabilities from low-income backgrounds through allied foundations. Commercialize prosthetic elements for low-income individuals. | *Start meeting: between the group of engineers from 3D engineering BQ and the CE CAMILO foundation to define the work team, design requirements, and types of patients for the project development. *Conceptual design development, prototype construction, and testing: through printing and testing with non-amputated people. *Patient interviews: in order to find suitable people for the use of prosthetics *Design, construction of customized prototype, and testing: and information is obtained *Redesign of the customized prototype: With the information found, a biomechanical study is carried out and the customized prototype is redesigned. *Construction of final prosthesis: through 3D printing of lower limb prosthesis, fully functional for people with low resources disabilities. *Prosthesis evaluation: where the user tests the final prosthesis in their day-to-day life and in control tests in the CE CAMILO laboratory. *Identification and Obtaining permits for distribution: in Colombia for the manufacture and distribution of prostheses and prosthetic elements. *Establishment of links, protocols, and procedures: with regional and national foundations and laboratories that work with people with disabilities and organizations that wish to donate resources. Procedures and protocols are established. *Prosthesis donation: A web platform is developed and social networks and media are used to contact people with disabilities with limited resources who wish to acquire prostheses, medical evaluation is carried out at the nearest partner foundation to their home, and prosthetic elements are sent for the manufacture of the prosthesis and the subsequent rehabilitation process of the person. *Prosthesis commercialization: Market research, marketing plan, and business plan are carried out for the commercialization of lower limb prostheses and the product is sold. Part of the resources obtained from the commercialization will be used for the donation of prostheses. | *Ideal patients were obtained for upper and lower limb. *A 3D scanner kinet for Windows, and CAD software meshmixer/solidwork and orthopedic rules (volume reduction and support fins) were used, and 3D printing (FFF) of ABS, to obtain a lower socket. It is compared with a conventionally manufactured socket and it is concluded that it reduces materials and number of stages, but it would be necessary to automate the process to be even more competitive in time and patient fit. *For the lower limb patient, developed prototypes of prosthetic knee and foot using simulation and finite element analysis with Solidwork, supported loads during walking test on horizontal plane, inclined plane, and steps, exhibited appropriate dynamic and biomechanical behavior. *For the upper limb, several versions were developed, a mechanical one with strong grip, fine grip and wrist rotation, a computer-operated robotic one with Arduino board and servo motors, a version controlled by a glove with sensors, and a final myoelectric prototype connected with electrodes to the patient's stump, 3D printed housing/arm, and conventional socket, which operated properly in strong grip, fine grip, and typing mode. *Cosmetic cover concepts for lower prostheses were developed and manufactured by 3D printing, personalized with anatomical shape emulating a real leg, and customizable shaped tattoos were included. | It was possible to develop upper and lower prostheses whose final cost was much lower than conventional prostheses. Their performance in patient tests was satisfactory. The current prototypes have appropriate biomechanical behavior suitable for patient use, and they are the basis for further improvement in order to meet national and international requirements for commercialization purposes. A market study was conducted with patients, orthopedic doctors, orthopedic laboratories, purchase and sale of orthopedic elements, and a commercial validation of the product was carried out, quantifying the demand and supply, sales prices and operating costs, analyzing the economic and financial proposal and business model, verifying its sustainability and viability. The developed cosmetic cover was positively received by patients, who highlighted the potential to make the prosthesis look more like a leg than a prosthesis. | Legal Aspects | Registered with INVIMA along with compliance with other workplace conditions. | For local marketing, INVIMA registration is required, which requires compliance with minimum manufacturing site conditions and good manufacturing practices system. | I estimated from market studies in orthopedic laboratories (potential clients) by interviewing technical managers, orthopedic technologists, physiatrists, and direct consultation with INVIMA. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME80 | Data: *Interview patients/clients in order to extract their tastes and personalize the design. *Develop the concepts in order to exceed client expectations, and ensure manufacturing in FFF. *Manually manufacture the concepts. *Select the best cover designs. *Manufacture the covers with 3D printing, and perform tests on coatings and paints. | The subject teacher is met to define the general guidelines of the project, which are communicated to the different work groups. The work groups interview the patients in order to personalize the client's preferences and incorporate them into the cover design. The groups develop the concepts and manually manufacture them. The evaluation jury, composed of the teacher and the researchers, judge based on general guidelines of aesthetics, concept, personalization of the patient's taste, manufacturability, among other criteria. The best designs are chosen, and they are modeled in 3D and manufactured using 3D printing with ABS material at a reduced scale. Subsequently, covers are manufactured at full scale and some covers are coated with transparent epoxy resin, and then painted with polyurethane paint to verify the aesthetics and final finish. | The students led by the classroom teacher develop and manually manufacture cosmetic covers that reflect the personal tastes of the patients. The best designs combine the best geometries, colors, and simplicity that facilitate and guarantee the feasibility of manufacturing through the 3D printing process. Subsequently, covers are manufactured on a reduced and natural scale, and tests are carried out with resins and paints, further improving the finish and appearance of the covers, obtaining the best results for epoxy coatings and polyurethane paints. | It is concluded that industrial design adds high added value to the covers, not only by reflecting specific tastes of patients by customizing the covers, but also by developing concepts beyond patients' expectations. The combination of colors and geometry gives a better appearance to the cover as a product and ensures simplicity in manufacturing. The application of epoxy resins and polyurethane paints improves the finishes of the product. | see ME81 | see ME81 | see ME81 | see ME81 |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Aesthetic | It should be aesthetic, with low surface roughness, and according to the patient's criteria: it includes conventional shapes and colors similar to the patient's original limb; and on the other hand, extravagant shapes with striking colors, and low relief tattoos according to the patient's taste. | As a master translator, I have translated the provided data into English. Here is the As for the conventional shape, it was designed with reference to the measurements and curved shapes of a normal leg. The cover was printed in ABS using FFF and polished with sandpaper to reduce roughness, apply epoxy resin, and paint with polyurethane. As for the extravagant and personalized shapes, in addition to the conventional shape, customized designs are used, whose concepts were inspired by the tastes of the patients but developed by industrial design students. | Over time, several covers are printed at full scale and different combinations are tested on each of them, without polishing, with tool polishing, manual sandpaper polishing, with epoxy resin, flexible resin, with paint on the resin, with paint without resin, and the texture and appearance result is judged. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Customization | The cover incorporates the colors, measurements, and external curves of the patient's leg, as well as the size. In the most demanding case, a particular design is included, with striking colors and tattoos in low relief or passing. | There are a variety of direct and indirect processes that use 3D printing, combined with sanding, painting, epoxy resin, thermoforming of laser-cut sheets. The specific process depends on the application of the cover, with 3D printing combined with painting being ideal for customization with direct patient measurements and modification of parametric design according to patient measurements, as long as the patient's physical activity is not demanding. | The use of 3D printing is crucial to achieve customization, through patient measurements and parametric design modification, but it must be supported by finishing processes such as sanding and painting. In case of requiring greater impact resistance, other manufacturing processes that combine 3D printing with thermoforming are recommended, but it increases the cost, unless the patient is satisfied with a standardized cover by the sizing system. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Dimensions/Ergonomics | The outer dimensions must match the patient's size. | The patient's measurements are taken and a sizing system is used, modifying a parametric design according to the patient's measurements. In the case of larger sizes, manufacturing by parts is considered, or the acquisition of larger printers or laser cutters. | It should be the same size as the patient. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Forces | It must be able to withstand strong, moderate, and light impacts according to the level of physical activity. | The specific manufacturing process and materials used depend on the level of physical activity: for strong impacts, PETG/Conventional High Impact Polystyrene sheet or 3D printed PLA sheet is recommended, both cases thermoformed with a 3D printed ABS mold; for intermediate impact and forces, the cover can be 3D printed with ABS, with minimum resistant thickness, reduction of stress concentrators, and epoxy resin to reinforce interlaminar impact resistance. | Impact tests are carried out with falls and hammer blows on printed test specimens manufactured in different orientations, applying different types of resin to a portion of the samples. The same test is performed for reduced-scale covers, full-scale covers, and covers with thicker wall thickness and reduced concentrators. The printed covers are field tested with patients. In the final stage, other manufacturing processes are tested that indirectly combine 3D printing to avoid the interlaminar weakness inherent in the 3D printing process. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Deformations/ movements | Deformations are not desirable for patients, nor are movements. | The thickness of the material and the material itself influence flexibility. In the case of PETG, wall thicknesses above 3mm are recommended. For high impact polystyrene and ABS/PLA, thicknesses between 2mm and 3mm are recommended. | After the covers were manufactured, they were subjected to manual static loads to corroborate the flexibility of the cover. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Materials | The materials must withstand the impact loads to which they are subjected, the static loads must not deform it appreciably or visibly, the texture of the material must guarantee the application of processes to improve the appearance such as sanding and the application of resins and paints. | The provided data has been translated into English: PETG/High impact polystyrene sheets were selected, cut by laser and thermoformed in an ABS printed mold. ABS printed directly in 3D was selected, as well as PLA printed directly in 2D and thermoformed by 3D molds in ABS. Depending on the thickness, the materials behave rigidly. All materials can be painted, but only ABS can be sanded directly. | An experimental process on specimens and covers, and field tests with the patient, allows us to conclude the influence of interlaminar impact resistance of printed covers as a limitation in the functionality of the cover under loads, promoting the use of materials with high impact resistance, coatings, and thermoforming process to avoid interlaminar weakness of printed material. Depending on the thickness, materials behave rigidly. All materials can be painted, but only ABS is susceptible to direct sanding. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Manufacturing and Assembly | Minimize direct personnel contact. The size or size of the cover, the printer, and laser cutter, and its speed limits the number of parts per cover assembly, as well as the number of printers/cutters to meet demand. The demand for prostheses in the country was estimated at 1000 units per year. | For direct 3D printing, contact is necessary and limited to sanding, epoxy resin application, and painting processes. For indirect thermoforming processes, physical contact is much greater. Depending on the size of the cover and the volume of the printer and cutter, the cover will be manufactured in multiple parts or as a single piece. Similarly, the monthly/annual production will determine the number of printers and cutters required to meet the demand. The demand for prosthetics in the country was estimated at 1000 units per year. In the case of 3D printing, around 10 printers would be required. | The type of manufacturing is dependent on the desired level of customization and physical activity requirements of the patient. For maximum customization and low physical activity, 3D printing is ideal and only requires direct contact during painting. The size and production of the cover will control the necessary sizes of printers and cutters and their number in the company. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | assembly | It should be easy and quick to attach to conventional prostheses, and the fit should be snug without any looseness or play or movement, or noticeable noises. | Bolted joints have proven to be effective in terms of their mechanical strength and fit, but they are not quick to attach to the prosthesis. | The glued joints proved to be inefficient in terms of mechanical strength and adjustments, the bolted joints demonstrated efficiency in mechanical strength and sufficient adjustment, but they are not quick to couple, the magnetic joints have not been designed or tested yet. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Service Life and Maintenance | Doctors recommend changing prostheses every 3 years, the international ISO standard 10328:2006 specifies the life of prostheses according to load cycling or number of steps (2 million cycles) and maintenance involves adjustments in screws and angles in pyramids. | Expected lifespan ranges from 1 year for printed materials to 3 years for conventional materials. | Printed materials have lower fatigue and impact resistance than conventional materials. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Costs and Deadlines | The cost of selling similar products is over 250 USD, the demand for prosthetics in the country was estimated at 1000 units per year. | The case of directly printed covers it is possible to achieve minimum market selling prices, achieving higher prices for fully customized covers. For the combination of indirect printing and thermoforming processes, costs can be much lower than those of printed covers, being more competitive if the sale is made by size. The times for size-based sales, for direct printing, are 4 to 5 days, and for laser cutting and thermoforming 1 to 2 days. For custom design, the time depends on the degree and difficulty of customization, adding an additional 1 to 3 days. Note: The translated value has been provided without any extra quotation or double quotation marks at the start or end. | During product validation and market research, the selling price of cosmetic covers in the market was consulted and compared with the quoted price of the 3D printed cover from the developing company. The quantity of units was estimated from a business model, along with its respective financial analysis, cost analysis, and profits, taking into consideration the manufacturing costs of the company, as well as the demand for upper and lower limb prosthetics in the country based on data from reputable national and international centers, and market studies from local orthopedic laboratories. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Security | The translated value of the provided data Los materiales no deben reaccionar con el cuerpo humano in English is Materials should not react with the human body. | PETG, PLA, ABS and Polystyrene are used in packaging applications for food and toys for children. | PETG is used in water bottles and food packaging in general, ABS and Polystyrene are used in Lego pieces and toys for children, PLA is non-toxic and therefore bio-safe. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME81 | Develop different covers using different manufacturing processes that use 3D printing as a support. Test the different cover concepts through resistance tests, and validation with clients and users. Develop joints to attach the covers to the prostheses. Test the resistance and fit of the joints. | Data: Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. Different cosmetic cover concepts are designed and manufactured using different manufacturing processes: direct 3D printing, thermoforming of printed materials, indirect thermoforming by 3D printing, combination with epoxy coatings, epoxy painting, and combinations. Tests are carried out on reduced scale covers, full-scale covers, field tests with the patient, and commercial validation with potential customers, in order to define the final resistance, size, and appearance. Different methods of attaching the cover to the prosthesis are designed and manufactured: glued joints, screwed joints, among others. The resistance of the joints is tested. | The most economical and fastest manufacturing process is the use of indirect thermoforming by 3D printing with ABS, combined with laser cutting of PETG/High Impact Polystyrene sheet, and the use of standard sizes. The resulting PETG cover does not exhibit the interlaminar weakness of conventionally manufactured printed materials. This cover is ideal for patients/customers with high physical activity, including jumps, falls, and impacts with blunt objects. Among the limitations, it shows an increase in labor and manual work, as well as the presence of a mold, and the restriction of handling size, which reduces customization. The manufacturing process of cosmetic covers by direct 3D printing proved to be feasible for a sufficient wall thickness, reduction of stress concentrators, and the combination of xtc 3d epoxy resin to withstand loads and impacts that would otherwise fracture the ABS cover due to interlaminar weakness. However, it is recommended for patients with intermediate physical activity such as small falls and mild impacts. Another additional advantage is customization without excessive additional costs, but there is a limitation in the manufacturing size dependent on the printer size, which would require in some cases manufacturing in parts. A last intermediate case between the previous two is the thermoforming of PLA printed material, but it is limited by the printing area that limits the developed surface to be printed. It has the advantage that it is printed in a strong printing direction, eliminating the interlaminar weakness defect. However, like the first process, it requires direct intervention and manual work for thermoforming, in addition to the mold and limitations in customization associated with the printing area and specific size. In general, more development is required for the cover fastenings, which do not have sufficient strength. | In general, it can be concluded that there are different methods of manufacturing covers using directly or indirectly 3D printing, each with its advantages and disadvantages and specific application according to the physical activity of the type of patient. | Legal Aspects | Unlike prosthetic elements, it does not require INVIMA permission. | As a master translator, I have translated the provided data into English. Here is the Since it does not interfere with the function of the prosthesis, it does not require INVIMA permission. | As a master translator, I have translated the provided data into English. Here is the Since it is not a direct element of use by the person with disabilities, but rather it is used on the prosthesis without interfering with its functionality, it does not require a special sales permit from INVIMA. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | *Designs and test selection for materials mechanics characterization. *Purchase of material for specimen manufacturing.*Manufacturing of specimens. *Execution of tests. *Preparation of materials testing report. *Definition of initial technical specifications for foot design. *Generation of alternatives and selection of foot design concepts. *Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic foot using CAD software.*Simulation of loads on prosthetic foot to optimize geometry based on structural integrity using Engineering software.*Preparation of design report with calculation notes. *Definition of initial technical specifications for knee design.*Generation of alternatives and selection of knee design concepts.*Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic knee using CAD software.*Simulation of loads on prosthetic knee to optimize geometry based on structural integrity using Engineering software. *Preparation of design report with calculation notes.*Purchase of materials for prototype manufacturing.*Purchase of standardized materials.*Manufacturing of non-standardized parts.*Assembly of prosthetic elements.*Selection of test subjects.*Clinical tests in gait laboratory.*Analysis of gait test results.*Preparation of gait test result report.*Quotation of standardized testing service for prosthetic elements.*Execution of prosthetic element test.*Preparation of report on standardized certification of prosthetic element test. *Technological transfer of the project through the hiring of SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the flexion test due to the anisotropic behavior of the 3D printed material. The final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure efforts through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Function | It should be able to support static and dynamic loads without failing for a specific period of time, and it should be able to absorb the impacts of walking and adapt to standardized elements. | Dynamic type prosthetic foot is chosen, TPU material with hardness of 75D or between 90-100 Shore A is chosen, loads and deformations are simulated, analyzed by finite element method, for different geometries similar to prosthetic feet in the market. | The type of foot and general geometry is chosen based on different types of feet available in the market, and criteria of economy, reliability, mobility, comfort, assemblability, standardization based on the initial specifications of ISO 10328 standard, cost, user characteristics, and compatibility. Materials printed in different manufacturing orientations of the specimens were characterized according to ASTM D638 standard, the properties of the materials were used to simulate the load in SolidWorks until it met the requirements of deformation and strength. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | *Designs and test selection for materials mechanics characterization. *Purchase of material for specimen manufacturing.*Manufacturing of specimens. *Execution of tests. *Preparation of materials testing report. *Definition of initial technical specifications for foot design. *Generation of alternatives and selection of foot design concepts. *Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic foot using CAD software.*Simulation of loads on prosthetic foot to optimize geometry based on structural integrity using Engineering software.*Preparation of design report with calculation notes. *Definition of initial technical specifications for knee design.*Generation of alternatives and selection of knee design concepts.*Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic knee using CAD software.*Simulation of loads on prosthetic knee to optimize geometry based on structural integrity using Engineering software. *Preparation of design report with calculation notes.*Purchase of materials for prototype manufacturing.*Purchase of standardized materials.*Manufacturing of non-standardized parts.*Assembly of prosthetic elements.*Selection of test subjects.*Clinical tests in gait laboratory.*Analysis of gait test results.*Preparation of gait test result report.*Quotation of standardized testing service for prosthetic elements.*Execution of prosthetic element test.*Preparation of report on standardized certification of prosthetic element test. *Technological transfer of the project through the hiring of SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Aesthetic | You must simulate the appearance of a real foot | A cosmetic cover was designed and manufactured in 75 shore A TPU. | The entire prosthetic foot cover was designed in a parametric format in SolidWorks that allows for scaling or measurement changes, and it was manufactured in 3D printing with flexible TPU material so that it can be adjusted to shoes and the prosthetic foot. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Customization | You must simulate the appearance of a real foot | A cosmetic cover was designed and manufactured in 75 shore A TPU. | The designed cover of the entire prosthetic foot was created in a parametric format in SolidWorks that allows for scaling or measurement changes, and it was manufactured using 3D printing with flexible TPU material so that it can be adjusted to shoes. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | *Designs and test selection for materials mechanics characterization. *Purchase of material for specimen manufacturing.*Manufacturing of specimens. *Execution of tests. *Preparation of materials testing report. *Definition of initial technical specifications for foot design. *Generation of alternatives and selection of foot design concepts. *Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic foot using CAD software.*Simulation of loads on prosthetic foot to optimize geometry based on structural integrity using Engineering software.*Preparation of design report with calculation notes. *Definition of initial technical specifications for knee design.*Generation of alternatives and selection of knee design concepts.*Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic knee using CAD software.*Simulation of loads on prosthetic knee to optimize geometry based on structural integrity using Engineering software. *Preparation of design report with calculation notes.*Purchase of materials for prototype manufacturing.*Purchase of standardized materials.*Manufacturing of non-standardized parts.*Assembly of prosthetic elements.*Selection of test subjects.*Clinical tests in gait laboratory.*Analysis of gait test results.*Preparation of gait test result report.*Quotation of standardized testing service for prosthetic elements.*Execution of prosthetic element test.*Preparation of report on standardized certification of prosthetic element test. *Technological transfer of the project through the hiring of SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the flexion test due to the anisotropic behavior of the 3D printed material. The final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure efforts through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Dimensions | According to the provided data, Acorde con la talla del paciente translates to According to the patient's size in English. | It was designed with a similar size to that of the patient and was manufactured. | It was designed with a similar size to that of the patient and was manufactured. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | *Designs and test selection for materials mechanics characterization. *Purchase of material for specimen manufacturing.*Manufacturing of specimens. *Execution of tests. *Preparation of materials testing report. *Definition of initial technical specifications for foot design. *Generation of alternatives and selection of foot design concepts. *Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic foot using CAD software.*Simulation of loads on prosthetic foot to optimize geometry based on structural integrity using Engineering software.*Preparation of design report with calculation notes. *Definition of initial technical specifications for knee design.*Generation of alternatives and selection of knee design concepts.*Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic knee using CAD software.*Simulation of loads on prosthetic knee to optimize geometry based on structural integrity using Engineering software. *Preparation of design report with calculation notes.*Purchase of materials for prototype manufacturing.*Purchase of standardized materials.*Manufacturing of non-standardized parts.*Assembly of prosthetic elements.*Selection of test subjects.*Clinical tests in gait laboratory.*Analysis of gait test results.*Preparation of gait test result report.*Quotation of standardized testing service for prosthetic elements.*Execution of prosthetic element test.*Preparation of report on standardized certification of prosthetic element test. *Technological transfer of the project through the hiring of SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the flexion test due to the anisotropic behavior of the 3D printed material. The final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure efforts through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Forces | According to Standard ISO 10328:2006, Comply with the criteria of resistance and durability of the standard, which implies supporting a static load of 3098N at a speed of 100N/s up to 250N/s, and supporting a dynamic load of 1230N for 2x10^6 cycles. Maximum weight of the foot 640gr. | Dynamic type prosthetic foot is chosen, TPU material with hardness shore 75D or between 90-100Shore A is chosen, loads and deformations are simulated and analyzed by finite element method, for different geometries similar to prosthetic feet in the market. | Materials printed in different manufacturing orientations of the test specimens were characterized according to ASTM D638 standard, the material properties were used to simulate the load in SolidWorks until it met the requirements of deformation and strength. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | *Designs and test selection for materials mechanics characterization. *Purchase of material for specimen manufacturing.*Manufacturing of specimens. *Execution of tests. *Preparation of materials testing report. *Definition of initial technical specifications for foot design. *Generation of alternatives and selection of foot design concepts. *Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic foot using CAD software.*Simulation of loads on prosthetic foot to optimize geometry based on structural integrity using Engineering software.*Preparation of design report with calculation notes. *Definition of initial technical specifications for knee design.*Generation of alternatives and selection of knee design concepts.*Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic knee using CAD software.*Simulation of loads on prosthetic knee to optimize geometry based on structural integrity using Engineering software. *Preparation of design report with calculation notes.*Purchase of materials for prototype manufacturing.*Purchase of standardized materials.*Manufacturing of non-standardized parts.*Assembly of prosthetic elements.*Selection of test subjects.*Clinical tests in gait laboratory.*Analysis of gait test results.*Preparation of gait test result report.*Quotation of standardized testing service for prosthetic elements.*Execution of prosthetic element test.*Preparation of report on standardized certification of prosthetic element test. *Technological transfer of the project through the hiring of SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Deformations | Motor skills, Mobility level, Minimum foot deflection of 25mm in the forefoot and 13mm in the heel at 1230N, Minimum energy absorption level of 75% in the forefoot and 83% in the heel. | Dynamic type prosthetic foot is chosen, TPU material with hardness shore 75D or between 90-100Shore A is chosen, loads and deformations are simulated and analyzed by finite element method, for different geometries similar to prosthetic feet in the market. | Materials printed in different manufacturing orientations of the test specimens were characterized according to ASTM D638 standard, the material properties were used to simulate the load in SolidWorks until it met the requirements of deformation and strength. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | *Designs and test selection for materials mechanics characterization. *Purchase of material for specimen manufacturing.*Manufacturing of specimens. *Execution of tests. *Preparation of materials testing report. *Definition of initial technical specifications for foot design. *Generation of alternatives and selection of foot design concepts. *Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic foot using CAD software.*Simulation of loads on prosthetic foot to optimize geometry based on structural integrity using Engineering software.*Preparation of design report with calculation notes. *Definition of initial technical specifications for knee design.*Generation of alternatives and selection of knee design concepts.*Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic knee using CAD software.*Simulation of loads on prosthetic knee to optimize geometry based on structural integrity using Engineering software. *Preparation of design report with calculation notes.*Purchase of materials for prototype manufacturing.*Purchase of standardized materials.*Manufacturing of non-standardized parts.*Assembly of prosthetic elements.*Selection of test subjects.*Clinical tests in gait laboratory.*Analysis of gait test results.*Preparation of gait test result report.*Quotation of standardized testing service for prosthetic elements.*Execution of prosthetic element test.*Preparation of report on standardized certification of prosthetic element test. *Technological transfer of the project through the hiring of SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Materials | The material must have enough strength to withstand the loads according to the standard, but it must have enough flexibility to absorb the impacts of the march. | Dynamic type prosthetic foot is chosen, TPU material with hardness shore 75D or between 90-100Shore A is chosen, loads and deformations are simulated and analyzed by finite element method, for different geometries similar to prosthetic feet in the market. | The initial selection was made based on properties reported by FFF/FDM material manufacturers. A weighted average matrix was used to select based on criteria of strength and flexibility. The printed materials were characterized in different manufacturing orientations of the test specimens according to ASTM D638 standard. The material properties were used to simulate the load in Solidworks until the geometry met deformation and strength requirements. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | *Designs and test selection for materials mechanics characterization. *Purchase of material for specimen manufacturing.*Manufacturing of specimens. *Execution of tests. *Preparation of materials testing report. *Definition of initial technical specifications for foot design. *Generation of alternatives and selection of foot design concepts. *Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic foot using CAD software.*Simulation of loads on prosthetic foot to optimize geometry based on structural integrity using Engineering software.*Preparation of design report with calculation notes. *Definition of initial technical specifications for knee design.*Generation of alternatives and selection of knee design concepts.*Selection of material and manufacturing process for non-standardized elements.*Selection of standardized mechanical components.*Geometric modeling of prosthetic knee using CAD software.*Simulation of loads on prosthetic knee to optimize geometry based on structural integrity using Engineering software. *Preparation of design report with calculation notes.*Purchase of materials for prototype manufacturing.*Purchase of standardized materials.*Manufacturing of non-standardized parts.*Assembly of prosthetic elements.*Selection of test subjects.*Clinical tests in gait laboratory.*Analysis of gait test results.*Preparation of gait test result report.*Quotation of standardized testing service for prosthetic elements.*Execution of prosthetic element test.*Preparation of report on standardized certification of prosthetic element test. *Technological transfer of the project through the hiring of SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Manufacturing and Assembly | The demand for prosthetics in the country was estimated at 1000 units per year. | The demand for prostheses in the country was estimated at 1000 units per year. In the case of 3D printing, about 10 printers would be required. | The estimated number of prostheses was determined based on market studies in orthopedic laboratories (potential clients) by interviewing technical managers, orthopedic technologists, physiatrists, and using information from population censuses to extrapolate local figures to the rest of the country. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | assembly | Assembly ease, Maximum assembly time 10 min. Standardization, Adaptability with market elements, Standard male-female pyramid couplings, standard tube. | Standard elements are selected to facilitate interchangeability and use by any orthopedic workshop, assembly time is also selected in 10 minutes. | The recommendation of standard elements is from the orthopedic technician. The assembly time is based on the experience of the project developers and orthopedic technicians. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Useful Life and Maintenance | support a dynamic load of 1230N for 2x10^6 cycles. | Expected An expected lifespan of between 1 year (2 x 10^6 cycles) for printed materials to 3 years for conventional materials. | Printed materials have lower fatigue and impact resistance than conventional materials. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Costs and Deadlines | Maximum manufacturing cost of element 1'000.000 COP or 250 USD. | Maximum manufacturing cost of element 1'000.000 COP or 250 USD. | Market prices are studied and the lowest price is chosen as the upper limit. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Ergonomics | Sizes are managed by patient sizes. | It was designed with a similar size to that of the patient and was manufactured. | It was designed with a similar size to that of the patient and was manufactured. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Legal Aspects | According to Standard ISO 10328:2006, Comply with the criteria of resistance and durability of the standard, and invima registration along with compliance with other workplace conditions. | Standardized tests are performed in a certified laboratory. | The standard is analyzed, the different tests are synthesized, the necessary tools are managed to reproduce the tests according to the standard, and the test is performed. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Function | It should be able to withstand static loads without failing for a specific period of time, and it should be able to move to simulate knee movement. | Knee polycentric is chosen (4-bar mechanism), Polycarbonate/Nylon 6 6 material is chosen, loads and deformations are simulated, analyzed by finite element method, for different geometries. | The type of knee and general geometry is chosen based on different types of knees available in the market, and criteria of economy, reliability, drivability, comfort, assemblability, standardization based on the initial specifications of ISO 10328 standard, cost, user characteristics, and compatibility. The printed materials were characterized in different manufacturing orientations of the specimens according to ASTM D638 standard, the material properties were used to simulate the load in SolidWorks until it met the requirements of deformation and strength. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Dimensions | According to the provided data, Acorde con la talla del paciente translates to According to the patient's size in English. | It was designed with a similar size to that of the patient and was manufactured. | It was designed with a similar size to that of the patient and was manufactured. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Movements | Mobility, Level of Mobility, knee rotation angle greater than 120°, Have knee locking position | Polycentric knee is chosen (4-bar mechanism), Policarbonate/Nylon 6 6 material is chosen, loads and deformations are simulated, analyzed by finite element method, for different geometries. | The dimensions and centers of the 4-bar mechanism were taken similar to an existing knee in the market, the correct operation was corroborated in SolidWorks simulation and in tests on patients. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | - | - | - | - |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | - | - | - | - |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Forces | Reliability, Resistance parameters of Standard ISO 10328:2006, Comply with the resistance and durability criteria of the standard, which implies withstanding a static load of 1750N in the locked position at a speed of 100N/s to 250N/s and Maximum bending resistance of 1750N. Comfort, Weight of the element, Maximum weight of the knee element 890gr. | Polycentric knee is chosen (4-bar mechanism), Policarbonate/Nylon 6 6 material is chosen, loads and deformations are simulated, analyzed by finite element method, for different geometries. | Materials printed in different manufacturing orientations of the test specimens were characterized according to ASTM D638 standard, the material properties were used to simulate the load in SolidWorks until it met the requirements of deformation and strength. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Materials | The material must have sufficient strength to withstand the loads according to the standard, and must be rigid to operate without deformation during operation. | Knee polycentric is chosen (4-bar mechanism), Polycarbonate/Nylon 6 6 material is chosen, loads and deformations are simulated, analyzed by finite element method, for different geometries. | The initial selection was made based on properties reported by FFF/FDM material manufacturers. A weighted average matrix was used to select based on criteria of strength and rigidity. The printed materials were characterized in different manufacturing orientations of the test specimens according to ASTM D638 standard. The material properties were used to simulate the load in Solidworks until deformation and strength requirements were met. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Manufacturing and Assembly | The demand for prosthetics in the country was estimated at 1000 units per year. | The demand for prosthetics in the country was estimated at 1000 units per year. In the case of 3D printing, about 10 printers would be required. | The estimated number of prostheses was based on market studies in orthopedic laboratories (potential clients) interviewing technical heads, orthopedic technologists, physiatrists, and information from population censuses to extrapolate local figures to the rest of the country. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | assembly | Assembly ease, Maximum assembly time 10 min. Standardization, Adaptability with market elements, Standard male-female pyramid couplings, standard tube. | Standard elements are selected to facilitate interchangeability and use by any orthopedic workshop, assembly time is also selected in 10 minutes. | The recommendation of standard elements is from the orthopedic technician. The assembly time is based on the experience of the project developers and orthopedic technicians. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Useful Life and Maintenance | support a dynamic load of 1230N for 2x10^6 cycles. | Expected An expected lifespan of between 1 year (2 x 10^6 cycles) for printed materials to 3 years for conventional materials. | Printed materials have lower fatigue and impact resistance than conventional materials. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Costs and Deadlines | Economy, Cost of the prosthetic element, Maximum manufacturing cost of the element 1'000.000 COP or 250 USD. | Maximum manufacturing cost of element 1'000.000 COP or 250 USD. | Market prices are studied and the lowest price is chosen as the upper limit. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | - | - | - | - |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Ergonomics | Sizes are managed by patient sizes. | It was designed with a similar size to that of the patient and was manufactured. | It was designed with a similar size to that of the patient and was manufactured. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | ME82 | Characterization of materials for the manufacture of prosthetic elements. Design a dynamic technology prosthetic foot that meets the standards of the Colombian Technical Standard. Design a mechanical polycentric prosthetic knee that meets the standards of the Colombian Technical Standard. Manufacture of prototype prosthetic elements. Conduct clinical tests with end users of developed prosthetic elements. Perform mechanical tests required to meet the standards of the Colombian Technical Standard. Transfer the knowledge generated in the project on material characterization and prosthetic design methodology. | Designs and test selection for materials mechanics characterization. Purchase of material for specimen manufacturing. Manufacturing of specimens. Execution of tests. Preparation of material testing report. Definition of initial technical specifications for foot design. Generation of alternatives and selection of foot design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic foot using CAD software. Simulation of loads on prosthetic foot for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Definition of initial technical specifications for knee design. Generation of alternatives and selection of knee design concepts. Selection of material and manufacturing process for non-standardized elements. Selection of standardized mechanical components. Geometric modeling of prosthetic knee using CAD software. Simulation of loads on prosthetic knee for geometry optimization based on structural integrity using engineering software. Preparation of design report with calculation notes. Purchase of materials for prototype manufacturing. Purchase of standardized materials. Manufacturing of non-standardized parts. Assembly of prosthetic elements. Selection of test subjects. Clinical tests in gait laboratory. Analysis of gait test results. Preparation of gait test result report. Quotation of standardized testing service for prosthetic elements. Execution of prosthetic element test. Preparation of report on standardized certification of prosthetic element test. Technological transfer of the project through the hiring of a SENA apprentice to support the project. | Through 3D printing, 3D printed materials (FFF) were characterized. The dynamic and mechanical behavior of the prosthetic foot and knee under loads and conditions according to international ISO standard 10328:2006 was modeled and simulated using finite element analysis. Initial prototypes were developed using PC materials for the polycentric two-axis knee (M4B) and dynamic foot in TPU. Universal machine tests confirmed the correct dynamic behavior of the foot. In terms of static strength tests according to international ISO standard 10328:2006, the foot did not suffer any damage, but the knee broke during the bending test due to the anisotropic behavior of the 3D printed material. Final prototypes were designed and manufactured using the plastic injection process, selecting new materials according to the technical data sheet of injected materials, and verifying failure stresses through modeling and finite element analysis. The final materials used for the knee were nylon 6.6 and for the foot, TPU with a hardness of 75 Shore D (between 90 and 100 Shore A). The foot test according to the standard was successful according to theoretical/numerical modeling. The theoretical/numerical modeling of the knee is also successful (the test could not be performed due to local COVID-19 quarantine). | By means of 3D printing, it was possible to develop a prosthetic foot capable of passing the static tests of the international ISO 10328:2006 standard without suffering damage. As for the prosthetic knee, due to the anisotropy of the material, it experienced mechanical failure during the tests in the weak plane due to manufacturing. The redesign of final prototypes to be manufactured by plastic injection ensures that in the theoretical/numerical modeling, the elements do not suffer damage when subjected to tests according to the standard, and this was corroborated in reality for the foot. The manufacturing costs for plastic injection are much lower than those for FFF 3D printing, even at a production rate of 50 for the foot and 100 units for the knee. | Legal Aspects | According to Standard ISO 10328:2006, Comply with the criteria of resistance and durability of the standard, and invima registration along with compliance with other workplace conditions. | Standardized tests are performed in a certified laboratory. | The standard is analyzed, the different tests are synthesized, the necessary tools are managed to reproduce the tests according to the standard, and the test is performed. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic sockets is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Function/Customization | Socket lower prosthesis: Prostheses usually consist of a socket, a suspension mechanism, alignable components, joints such as knees or ankles, and a foot; Patient customization. | Scan the stump using a 3D laser scanner, modify the geometry using CAD software, and use 3D printing to produce the socket. with an attachment to fix the pylon. | Digital scanning ensures customization, and the conversion from surface to digital solid and modeling, through 3D modeling, ensures the rigidity, alignment, and support required for the patient, as well as reducing pressure on the stump. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Function/Customization | AFO (ankle and foot orthosis): used to support and align, suppress spastic and overloaded muscles, assist weak and paralyzed muscles, prevent or correct deformities, and improve ankle and foot functions; Patient customization. | The 3D scanning of the ankle and foot (including the plantar surface). The plantar surface of the subject's foot is also scanned, either using the foam impression box or by direct scanning. The scanning data is processed using Tracer® CAD and MagicsTM. Two scans can be stitched together by aligning three fixed points placed on the edge of the foam box, which are scanned during both scans. The trimming lines for the AFO were created and manually smoothed. A five-step manufacturing and customization framework was proposed based on subject characterization and alignment of the foot and leg segment using reference points. | Digital scanning ensures customization, and the conversion from surface to digital solid and modeling, through 3D modeling, ensures the required rigidity, alignment, and support for corrections in the ankle-foot. Clinically, AFO SLA (flexible and rigid) were tested and showed equivalent walking speed, step length, and double support time compared to standard AFO in the study of gait parameters. SLS and regular PP AFOs were studied in 8 subjects with unilateral foot drop gait. SLS and PP AFOs showed equivalent performances. Clinical evaluation was conducted on 10 subjects with unilateral lower limb impairments, measuring their gait using regular carbon fiber and matched rigidity SLS AFOs. Minimal differences in gait performance were observed. SLS AFOs can be applied to study the effects of design alterations on gait performance. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Function/Customization | FO (foot orthoses): support and align the foot to prevent or correct foot deformities, provide even distribution of body weight, or improve foot function; Patient customization | A 3D laser scanner captures the 3D geometry by scanning either the foam box print or directly scanning the patient's foot. Orthopedists make modifications to the geometry using Tracer® CAD. The modified geometry is exported to STL and transferred to another software, MagicsTM, to generate a solid model. In SolidWorksTM, a curing block is generated and exported as another STL file. These two STL files are merged in MagicsTM, creating a single STL file for manufacturing the FO. | Digital scanning ensures customization, and the conversion from surface to digital solid and modeling, through 3D modeling, ensures the rigidity, alignment, and support required for corrections in the foot. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Dimensions | Socket lower prosthesis: The volume of a stump gradually decreases due to muscle atrophy, reduction of edema, with daily and constant use of a prosthesis. The volume of the limb can also increase due to sores, salt intake, medication, or trauma. | Modifications are used to add volume to any bony prominence or sensitive areas and volume is removed from pressure tolerant areas, as determined by the assessment and experience of the prosthetist. Prosthetic socks of various thicknesses (ply) are commonly used inside the socket to compensate for volume loss in the patient's limb. | determined by the evaluation and experience of the prosthetist. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Dimensions | AFO (ankle and foot orthosis): The surface should be smooth both inside and in contact with the patient. | It is proceeded to finish by hand or explore and the effect of orientation in SLS on dimensional accuracy. | Through experimental process, the accuracy of the SLS was tested. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Forces/Deformations | Socket lower prosthesis: amputees suffer from the discomfort of high pressure points of contact in the socket. Associated with the volume change is the change in pressure and pressure points. The user feels discomfort at the distal end as a result of pressure on the tibia and/or other bony prominences. Improve durability. The volume of a stump gradually decreases due to muscle atrophy, reduction of edema, with daily and constant use of a prosthesis. The volume of the limb can also increase due to sores, salt intake, medication, or trauma. | They had integrated the compatible lace technology into a test to measure contact pressure. Cover the 3D printed laces with resin to improve durability. | Measurement pressure tests are carried out on the stump-socket interface. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Forces/Deformations | AFO (ankle and foot orthosis): It must have adequate rigidity and cushioning compared to conventional orthoses, in the same way the combination of force, geometry, and material must guarantee the non-destruction of the orthosis during treatment. | Finite element testing and simulations are carried out to compare conventional orthoses with those that would be additively manufactured. | They applied finite element modeling (FEM) and topology optimization for the design of AFOs manufactured by SLS. They integrated CAD model parameterization and FEM analysis to quantitatively refine and predict and experimentally validate the bending stiffness of the FDM AFO. They used SLS Nylon 12 filled with Nylon 12 and Nylon 11 fiberglass and tested it in three ways. The results were compared with a carbon fiber AFO and showed that SLS was ideal for manufacturing AFOs with adequate stiffness and better cushioning. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Forces/Deformations | FO (foot orthosis): Pressure distribution should be uniform and similar to conventionally manufactured FOs. | Walking and pressure measurement tests are decided to be carried out. Material and process are selected: Nylon 12 (PA-12) and SLS. | Through a pressure pad, the pressure and its distribution on the patient's limb are verified while walking, resulting in a pressure similar to conventional FO. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Materials | Lower limb socket prosthesis: Associated with volume change is the change in pressure and pressure points. The user feels discomfort at the distal end as a result of pressure on the tibia and/or other bony prominences. Improve durability. It will be in direct contact with the patient's stump, poor ergonomics and friction can generate health problems. | Design a lace with variable hardness using the Objet Connex capable of 3D printing plastic with 10 levels of polymer hardness. Cover the 3D printed laces with resin to improve durability. ABS FDM, Prosthetic socks of various thicknesses (ply) are usually used inside the lace to compensate for volume loss in the patient's limb. | The lace was designed in such a way that its hardness had an inverse relationship to the conformity of the fabric at each point of contact (i.e., the more conforming fabric rested against the harder material, and vice versa). |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Materials | AFO (ankle and foot orthosis): It must have adequate rigidity and cushioning compared to conventional orthoses. Similarly, the combination of force, geometry, and material must ensure the non-destruction of the orthosis during treatment. | Finite element testing and simulations are carried out to compare conventional orthoses with additively manufactured ones. Using Nylon 12 filled with Nylon 12 fiberglass and Nylon 11. ABS/PLA for FDM. | They applied finite element modeling (FEM) and topology optimization for the design of AFOs manufactured by SLS. They integrated CAD model parameterization and FEM analysis to quantitatively refine and predict and experimentally validate the bending stiffness of the FDM AFO. SLS was ideal for manufacturing AFOs with adequate stiffness and improved damping. Nylon 11 was the SLS material that could withstand the full range of destructive tests. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Materials | FO (foot orthosis): The materials should provide an even weight distribution. | PA-12, PLA/ABS Language: English PA-12, PLA/ABS | Through a pressure pad, the pressure and its distribution on the patient's limb are verified while walking, resulting in a pressure similar to conventional FO. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Manufacturing and Assembly | Socket lower prosthesis: Associated with the change in volume is the change in pressure and pressure points. The user feels discomfort at the distal end as a result of pressure on the tibia and/or other bony prominences. Improve durability. It will be in direct contact with the patient's stump, poor ergonomics and friction can generate health problems. | SLA/SLS/FDM, Objet conex (photocurable resin or multimateiral photopolymer), cover 3D printed joints with resin to improve durability. | The design criterion was the hardness of the tissue, and the durability of the material, although the cost of building and testing an SLA plug was investigated and the cost of potential application was studied. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Manufacturing and Assembly | AFO (ankle and foot orthosis): manufacturing, and costs should be competitive with conventional manufacturing processes. | FDM/SLS/SLA translates to FDM/SLS/SLA in ingles. | The FDM route for the AFO and the support structure was designed and the AFOs were manufactured and evaluated in the users. SLS was ideal for manufacturing AFO with adequate rigidity and better cushioning. Nylon 11 was the material of SLS that can withstand the full range of destructive tests. Through experimental process, the accuracy of SLS and the cost effectiveness of SLS vs. traditional AFO were tested. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Manufacturing and Assembly | FO (foot orthosis): manufacturing, and costs must be competitive with conventional manufacturing processes. | FDM/SLS translates to FDM/SLS in ingles. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | assembly | Lower limb socket prosthesis: alignable components, joints like knees or ankles, and a foot. | produce the lace. with an attachment to fix the pestle | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | assembly | AFO (ankle and foot orthosis): with adjustable stiffness levels in the sagittal plane to adjust stiffness at the ankle joint. | they showed the effect of stiffness on ankle kinematics in a healthy subject | Experimental test. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | assembly | FO (foot orthosis): It must be adjustable during treatment (clubfoot, rheumatoid arthritis) | Reducing the maximum pressure on the metatarsal heads using adjustable elements. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Costs and Deadlines | Lower limb socket prosthesis: potential cost of application compared to other conventional methods and durability matching. | Coated SLS/FDM with resin | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Costs and Deadlines | AFO (ankle and foot orthosis): The design and manufacturing time, and costs should be competitive compared to conventional manufacturing processes (The duration of traditional manufacturing is usually two weeks). | Through experimental process, the accuracy of SLS and the cost effectiveness of SLS vs. traditional AFO were tested. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Costs and Deadlines | FO (foot orthosis): The design and manufacturing time, and costs must be competitive compared to conventional manufacturing processes (30 to 60 minutes The machining time of a standard size FO on the Amfit® CNC carver, not including time for pin contact digitizer) | The manufacturing time for FDM is 60 minutes susceptible to be optimized through topological optimization. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Ergonomics | Socket lower prosthesis: It will be in direct contact with the patient's stump, poor ergonomics and friction can generate health problems such as ulcerations and infections. | Design a lace with variable hardness using the Objet Connex capable of 3D printing plastic with 10 levels of polymer hardness. Cover the 3D printed laces with resin to improve durability. ABS FDM, Prosthetic socks of various thicknesses (ply) are usually used inside the lace to compensate for volume loss in the patient's limb. | The lace was designed in such a way that its hardness had an inverse relationship to the conformity of the fabric at each point of contact (i.e., the more conforming fabric rested against the harder material, and vice versa). |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Ergonomics | AFO (ankle and foot orthosis): custom manufacturing, and alignment, rigidity, and adjustable fit characteristics. It will be in direct contact with the patient's stump, poor ergonomics and friction can generate health problems such as ulcerations and infections. | A 3D laser scanner captures the geometry, is modified to solidify, provide alignment, rigidity and controllable fit, is manually finished or smoothed to reduce friction. | Digital scanning ensures customization, and the conversion from surface to digital solid and modeling, through 3D modeling, ensures rigidity, alignment rigidity, and controllable adjustment, manual finishing ensures smoothness to avoid friction. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Ergonomics | Custom foot orthosis: custom manufacturing, alignment features, and uniform support. | A 3D laser scanner captures the geometry, is modified to solidify, provide alignment and uniform support. | Digital scanning ensures customization, and the conversion from surface to digital solid and modeling, through 3D modeling, ensures the rigidity, alignment, and support required for corrections in the foot. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis, lower orthosis | 64 | Additive manufacturing (AM) of custom foot orthoses (FO), ankle-foot orthoses (AFO), and prosthetic socket is reviewed and compared to traditional techniques of plaster mold manufacturing. | First of all, a study was conducted at the Orthotics and Prosthetics Center of the University of Michigan (UMOPC) to investigate the quantity and revenue of various types of orthotics and prosthetics. The current manufacturing process at UMOPC and a detailed review of the design and AM research of these three types of orthotics and prosthetics in the last 25 years are being investigated. | The FO and AFO were identified as the highest income orthoses. The laborious steps and long manufacturing time of the traditional manufacturing method and the progress and benefits of AM for custom orthoses and prostheses are evident. | The study concludes that there are still clinical, financial, and technological barriers to the large-scale implementation of anthroposophic medicine in a personalized orthotics and prosthetics service system. | Legal Aspects | Lower limb socket prosthesis/AFO (ankle and foot orthosis)/FO (foot orthosis): involves appropriate medical facilities and good manufacturing practices system. | Study conducted at a specialized medical center. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | ME17 | The custom AFOs (ankle-foot orthoses) offer a better fit, comfort, and functional performance than the prefabricated ones. The 3D printing technique is ideal for manufacturing custom AFOs. Fused Deposition Modeling (FDM) is a 3D printing method with the desired material deposition strength and rate for custom AFO applications. Process planning is critical for cycle time and quality for FDM of AFOs. | Four steps in the process planning are: 1) determination of orientation, 2) support generation, 3) cutting, and 4) tool path generation. To reduce the support structure, structural optimizations are applied in the AFO part without compromising strength. Adaptive slicing strategy is used to slice the AFO with full consideration of its geometric characteristics (parameter optimization). | In determining the orientation, several factors are taken into account to improve the printability and mechanical performance of the manufactured AFO. In the generation of the tool path, the wavy tool path is developed to improve the manufacturing process and enhance structural strength through parameter optimization. | This study provided an overview of the FDM planning process of the AFO. Illustrating four critical steps in the planning process for FDM, the 3D model of the AFO from the scanned cloud data was processed step by step for FDM. | Function | Increase mechanical resistance to bending | Selecting the horizontal manufacturing orientation in order to increase bending strength. Wavy paths are proposed that increase bending strength by 6% compared to the traditional zigzag path (but require more development). | Through qualitative and comparative analysis (multicriteria matrix: number of layers, flexural resistance, support quantity, roughness), 3 orientations are proposed that minimize the staircase effect but increase resistance. The effects on the resistance zone by zone of the AFO are analyzed and the one that maximizes resistance is selected. The resistance to flexion is quantified through bending tests on specimens with different types of trajectories. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | ME17 | The custom AFOs (ankle-foot orthoses) offer a better fit, comfort, and functional performance than the prefabricated ones. The 3D printing technique is ideal for manufacturing custom AFOs. Fused Deposition Modeling (FDM) is a 3D printing method with the desired material deposition strength and rate for custom AFO applications. Process planning is critical for cycle time and quality for FDM of AFOs. | Four steps in the process planning are: 1) determination of orientation, 2) support generation, 3) cutting, and 4) tool path generation. To reduce the support structure, structural optimizations are applied in the AFO part without compromising strength. Adaptive slicing strategy is used to slice the AFO with full consideration of its geometric characteristics (parameter optimization). | In determining the orientation, several factors are taken into account to improve the printability and mechanical performance of the manufactured AFO. In the generation of the tool path, the wavy tool path is developed to improve the manufacturing process and enhance structural strength through parameter optimization. | This study provided an overview of the FDM planning process of the AFO. Illustrating four critical steps in the planning process for FDM, the 3D model of the AFO from the scanned cloud data was processed step by step for FDM. | For the given data Fuerzas/Materiales and the desired language ingles, the translation is Forces/Materials. | Increase mechanical resistance | Selecting the horizontal manufacturing orientation in order to increase bending strength. Wavy paths are proposed that increase bending strength by 6% compared to the traditional zigzag path (but require more development). | Through qualitative and comparative analysis (multicriteria matrix: number of layers, flexural resistance, support quantity, roughness), 3 orientations are proposed that minimize the staircase effect but increase resistance. The effects on the resistance zone by zone of the AFO are analyzed and the one that maximizes resistance is selected. The resistance to flexion is quantified through bending tests on specimens with different trajectory types. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | ME17 | The custom AFOs (ankle-foot orthoses) offer a better fit, comfort, and functional performance than the prefabricated ones. The 3D printing technique is ideal for manufacturing custom AFOs. Fused Deposition Modeling (FDM) is a 3D printing method with the desired material deposition strength and rate for custom AFO applications. Process planning is critical for cycle time and quality for FDM of AFOs. | Four steps in the process planning are: 1) determination of orientation, 2) support generation, 3) cutting, and 4) tool path generation. To reduce the support structure, structural optimizations are applied in the AFO part without compromising strength. Adaptive slicing strategy is used to slice the AFO with full consideration of its geometric characteristics (parameter optimization). | In determining the orientation, several factors are taken into account to improve the printability and mechanical performance of the manufactured AFO. In the generation of the tool path, the wavy tool path is developed to improve the manufacturing process and enhance structural strength through parameter optimization. | This study provided an overview of the FDM planning process of the AFO. Illustrating four critical steps in the planning process for FDM, the 3D model of the AFO from the scanned cloud data was processed step by step for FDM. | Manufacturing and Assembly | Reduce manufacturing times and improve finishing. | When selecting the Horizontal manufacturing orientation in FDM, the number of layers is reduced but the number of supports is increased. To reduce manufacturing time and material, the AFO is modified so that it no longer requires as much support. To control the height, wavy trajectories are proposed for extrusion, which reduces time by 23% compared to zigzag methods and reduces weight by 17% (but requires more development). | The reduction of parts of the AFO that are not required to support load also reduces the amount of area that requires support. The change of internal trajectory verified by simulation reduces printing times (but requires further development). |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | ME17 | The custom AFOs (ankle-foot orthoses) offer a better fit, comfort, and functional performance than the prefabricated ones. The 3D printing technique is ideal for manufacturing custom AFOs. Fused Deposition Modeling (FDM) is a 3D printing method with the desired material deposition strength and rate for custom AFO applications. Process planning is critical for cycle time and quality for FDM of AFOs. | Four steps in the process planning are: 1) determination of orientation, 2) support generation, 3) cutting, and 4) tool path generation. To reduce the support structure, structural optimizations are applied in the AFO part without compromising strength. Adaptive slicing strategy is used to slice the AFO with full consideration of its geometric characteristics (parameter optimization). | In determining the orientation, several factors are taken into account to improve the printability and mechanical performance of the manufactured AFO. In the generation of the tool path, the wavy tool path is developed to improve the manufacturing process and enhance structural strength through parameter optimization. | This study provided an overview of the FDM planning process of the AFO. Illustrating four critical steps in the planning process for FDM, the 3D model of the AFO from the scanned cloud data was processed step by step for FDM. | Costs and Deadlines | Reduce manufacturing times without sacrificing strength. | The AFO is modified so that it no longer requires as much support. Wavy trajectories are proposed for extrusion, which reduces time by 23% compared to zigzag methods, increases flexion strength by 6%, reduces weight by 17% (but requires more development). | The change of internal trajectory verified by simulation reduces printing times (but requires further development) |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | ME17 | The custom AFOs (ankle-foot orthoses) offer a better fit, comfort, and functional performance than the prefabricated ones. The 3D printing technique is ideal for manufacturing custom AFOs. Fused Deposition Modeling (FDM) is a 3D printing method with the desired material deposition strength and rate for custom AFO applications. Process planning is critical for cycle time and quality for FDM of AFOs. | Four steps in the process planning are: 1) determination of orientation, 2) support generation, 3) cutting, and 4) tool path generation. To reduce the support structure, structural optimizations are applied in the AFO part without compromising strength. Adaptive slicing strategy is used to slice the AFO with full consideration of its geometric characteristics (parameter optimization). | In determining the orientation, several factors are taken into account to improve the printability and mechanical performance of the manufactured AFO. In the generation of the tool path, the wavy tool path is developed to improve the manufacturing process and enhance structural strength through parameter optimization. | This study provided an overview of the FDM planning process of the AFO. Illustrating four critical steps in the planning process for FDM, the 3D model of the AFO from the scanned cloud data was processed step by step for FDM. | Ergonomics | Reduce the ladder effect, to reduce friction with the patient. | To control the layer height, the AFO is divided into zones and the layer height parameters are adjusted depending on the zone requirement. | The contact with the patient can cause other health problems, such as ulcers, abrasions, discomfort. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 192 | The possibility of conferring non-homogeneous properties to the product provides an important design key. This work refers to the design and manufacture of medical devices that require a high level of customization. The proposed method focuses on the virtual modeling of the patient's stump and the virtual process is highly integrated and the data flow is as smooth as possible. | . Three main phases can be identified: design, validation, and manufacture of the socket. First, the technician uses the Socket Modeling Assistant (SMA) tool to design the shape of the socket. Then, a numerical simulation is run to check the pressure distribution and validate the shape of the socket. Finally, a multimaterial 3D printer is used to build the socket. Preliminary results are presented, and conclusions are drawn about the challenge of 3D printing. | One way to gather and harmonize commercial and ad hoc tools to create a smooth flow of data. | This work contributes to the definition of a viable workflow for orthopedic technicians creating lower limb prostheses. The approach is to move towards a complete virtual approach that goes from magnetic resonance images to 3D printed fitting to be assembled with standard components. | Function/Customization | Prostheses usually consist of a socket, a suspension mechanism, alignable components, joints such as knees or ankles, and a foot; Patient customization. | The 3D model of the stump is generated from Magnetic Resonance Imaging (MRI) data. The socket modeling is possible through a custom CAD application called Socket Modeling Assistant (SMA). SMA virtualizes all the steps of the conventional socket manufacturing process around the 3D virtual model of the stump. | The module has been built by leveraging orthopedic knowledge extracted from interviews with doctors and the best cases, encoded and incorporated into the system. The first step is to provide the patient's history and data, such as weight, muscle tone, skin conditions, and stump stability, which will be used to apply embedded design rules and/or suggest the best approach for fitting. To modify the shape, there is a scaling tool, marks, deformation, top (to create the bottom part), sculpting, cut line (top part for load support), and valve hole (for the bottom part). |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 192 | The possibility of conferring non-homogeneous properties to the product provides an important design key. This work refers to the design and manufacture of medical devices that require a high level of customization. The proposed method focuses on the virtual modeling of the patient's stump and the virtual process is highly integrated and the data flow is as smooth as possible. | . Three main phases can be identified: design, validation, and manufacture of the socket. First, the technician uses the Socket Modeling Assistant (SMA) tool to design the shape of the socket. Then, a numerical simulation is run to check the pressure distribution and validate the shape of the socket. Finally, a multimaterial 3D printer is used to build the socket. Preliminary results are presented, and conclusions are drawn about the challenge of 3D printing. | One way to gather and harmonize commercial and ad hoc tools to create a smooth flow of data. | This work contributes to the definition of a viable workflow for orthopedic technicians creating lower limb prostheses. The approach is to move towards a complete virtual approach that goes from magnetic resonance images to 3D printed fitting to be assembled with standard components. | For the given data Fuerzas/Material and the desired language ingles, the translation is Forces/Material. | Amputees suffer from discomfort caused by high pressure points of contact in the socket. Associated with volume change is pressure change and pressure points. The user feels discomfort in the distal end as a result of pressure on the tibia and/or other bony prominences. Improve durability. The volume of a residual limb gradually decreases due to muscle atrophy, reduction of edema, with daily and constant use of a prosthesis. The volume of the limb can also increase due to sores, salt intake, medication, or trauma. | Pressure analysis: the platform incorporates a module specifically developed to integrate a numerical simulation tool and allow the prosthetist to automatically perform the FE analysis and validate the fit design and functionality. In order to have a safe product to test with patients and prevent accidents due to plug breakage, the filling ratio has been overestimated up to 70% with a honeycomb pattern. | The module has been built by leveraging orthopedic knowledge extracted from interviews with doctors and the best cases, encoded and incorporated into the system. Through numerical simulation, it is possible to evaluate the fit shape by analyzing the pressure at the residual-socket interface, as it is the main parameter commonly used for this purpose. Implement an automatic procedure to provide medical staff with the results of the FE simulation without the intervention of a simulation expert. The results of the FE simulation highlight eventual pressure peaks or other issues that can be passed and visualized within SMA to be automatically or interactively corrected. Initially, patients were asked to put on the socket and test the fitting phase, and then they were able to stand up and perform a static vertical load using a suitable support for the socket. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 192 | The possibility of conferring non-homogeneous properties to the product provides an important design key. This work refers to the design and manufacture of medical devices that require a high level of customization. The proposed method focuses on the virtual modeling of the patient's stump and the virtual process is highly integrated and the data flow is as smooth as possible. | . Three main phases can be identified: design, validation, and manufacture of the socket. First, the technician uses the Socket Modeling Assistant (SMA) tool to design the shape of the socket. Then, a numerical simulation is run to check the pressure distribution and validate the shape of the socket. Finally, a multimaterial 3D printer is used to build the socket. Preliminary results are presented, and conclusions are drawn about the challenge of 3D printing. | One way to gather and harmonize commercial and ad hoc tools to create a smooth flow of data. | This work contributes to the definition of a viable workflow for orthopedic technicians creating lower limb prostheses. The approach is to move towards a complete virtual approach that goes from magnetic resonance images to 3D printed fitting to be assembled with standard components. | assembly | It should allow assembly with valve and lower part of prosthesis | Tool program allows for the creation of a valve hole (for the bottom). | The valve is to improve the suction and adherence of the socket to the stump. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 192 | The possibility of conferring non-homogeneous properties to the product provides an important design key. This work refers to the design and manufacture of medical devices that require a high level of customization. The proposed method focuses on the virtual modeling of the patient's stump and the virtual process is highly integrated and the data flow is as smooth as possible. | . Three main phases can be identified: design, validation, and manufacture of the socket. First, the technician uses the Socket Modeling Assistant (SMA) tool to design the shape of the socket. Then, a numerical simulation is run to check the pressure distribution and validate the shape of the socket. Finally, a multimaterial 3D printer is used to build the socket. Preliminary results are presented, and conclusions are drawn about the challenge of 3D printing. | One way to gather and harmonize commercial and ad hoc tools to create a smooth flow of data. | This work contributes to the definition of a viable workflow for orthopedic technicians creating lower limb prostheses. The approach is to move towards a complete virtual approach that goes from magnetic resonance images to 3D printed fitting to be assembled with standard components. | Security | Prevent accidents due to breakage. | Patients and prevent accidents due to plug breakage, the filling ratio has been overestimated up to 70% with a honeycomb pattern. | Prevent any other type of harm or injury to the patient during walking there are no quotation or double quotation marks at the start or end of the translated value. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 123 | This work presents a cybernetic design and additive manufacturing system (CDAM) at the Center for Orthotics and Prosthetics at the University of Michigan. | The ankle and foot orthosis (AFO), a custom-made orthosis that supports muscles and provides control and protection for the feet, is selected as an example to demonstrate the CDAM system. Four key components: 1) digital scanning of the foot and leg geometry using a support with a transparent plate for the foot and an ergonomic procedure for 3D optical scanning, 2) cloud-based design software that allows clinicians to access scanned point cloud data on the geometry of patients' feet, as well as create 3D models for patient-based AFOs, 3) cloud-based manufacturing software that generates tool paths and process parameters for additive manufacturing to fabricate the AFO, 4) evaluation using the inertial measurement unit (IMU) for AFO motion measurement for gait analysis. | This CDAM system helps to shorten delivery and improve the fit and comfort of custom orthoses and prostheses. Traditionally, custom orthoses are made using plaster molds that require multiple patient visits and a lot of time and work for manufacturing. The feasibility of the CDAM system for one-day delivery of AFOs to patients has been demonstrated. | In this work, an application of a CDAM system that has been implemented for practical design and manufacturing, as well as for evaluations of patients with customized AFOs in a clinical environment at UMOPC, was presented. The potential of CDAM to improve clinical services for personalized AFOs and enhance the patient experience by allowing a one-day visit has been demonstrated. The ongoing research aims to demonstrate that this CDAM system is sustainable and reliable in operation. Furthermore, with the use of cloud computing, cybersecurity and privacy issues, including confidentiality, integrity, and data availability, are important for the CDAM system to address. | Function/Customization | To support and align, suppress spastic and overloaded muscles, help weak and paralyzed muscles, prevent or correct deformities, and improve ankle and foot functions; Patient customization. | Data: Data capture of 3D shapes: 3D optical scanning is performed using a support with a transparent and anti-reflective footplate, as well as an ergonomic scanning protocol for the physician to accurately, easily, and quickly scan the foot and leg for the AFO. Cybernetic design software for custom orthoses: Software guides clinicians to process the scanned surface data, modify the geometry, and define the cutting line based on patient needs. Surface geometry data is converted into three-dimensional data based on user usage and load conditions and FDMTM processing capability. This web-based software can utilize cloud computing capability. For some hospitals, internet access from the outside may be difficult, and a standalone version of the software can be used. The built-in Inertial Measurement Unit (IMU) in the AFO is used to collect motion data while the user is outside the clinic. Motion data is uploaded to the cybernetic design center and applied for gait analysis, health status prognosis, and better AFO design. Data capture of 3D shapes: 3D optical scanning is performed using a support with a transparent and anti-reflective footplate, as well as an ergonomic scanning protocol for the physician to accurately, easily, and quickly scan the foot and leg for the AFO. Cybernetic design software for custom orthoses: Software guides clinicians to process the scanned surface data, modify the geometry, and define the cutting line based on patient needs. Surface geometry data is converted into three-dimensional data based on user usage and load conditions and FDMTM processing capability. This web-based software can utilize cloud computing capability. For some hospitals, internet access from the outside may be difficult, and a standalone version of the software can be used. The built-in Inertial Measurement Unit (IMU) in the AFO is used to collect motion data while the user is outside the clinic. Motion data is uploaded to the cybernetic design center and applied for gait analysis, health status prognosis, and better AFO design. | Determined by the evaluation and experience of the prosthetist. Clinicians mark the bone areas and manipulate the STL geometry of the surface to increase or decrease the free space to meet the specific needs of the patients. It would be ideal to use Magnetic Resonance Imaging to better simulate the behavior of soft and bony tissues, optical procedures only focus on the exterior. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 123 | This work presents a cybernetic design and additive manufacturing system (CDAM) at the Center for Orthotics and Prosthetics at the University of Michigan. | The ankle and foot orthosis (AFO), a custom-made orthosis that supports muscles and provides control and protection for the feet, is selected as an example to demonstrate the CDAM system. Four key components: 1) digital scanning of the foot and leg geometry using a support with a transparent plate for the foot and an ergonomic procedure for 3D optical scanning, 2) cloud-based design software that allows clinicians to access scanned point cloud data on the geometry of patients' feet, as well as create 3D models for patient-based AFOs, 3) cloud-based manufacturing software that generates tool paths and process parameters for additive manufacturing to fabricate the AFO, 4) evaluation using the inertial measurement unit (IMU) for AFO motion measurement for gait analysis. | This CDAM system helps to shorten delivery and improve the fit and comfort of custom orthoses and prostheses. Traditionally, custom orthoses are made using plaster molds that require multiple patient visits and a lot of time and work for manufacturing. The feasibility of the CDAM system for one-day delivery of AFOs to patients has been demonstrated. | In this work, an application of a CDAM system that has been implemented for practical design and manufacturing, as well as for evaluations of patients with customized AFOs in a clinical environment at UMOPC, was presented. The potential of CDAM to improve clinical services for personalized AFOs and enhance the patient experience by allowing a one-day visit has been demonstrated. The ongoing research aims to demonstrate that this CDAM system is sustainable and reliable in operation. Furthermore, with the use of cloud computing, cybersecurity and privacy issues, including data confidentiality, integrity, and availability, are important for the CDAM system to address. | Forces/Materials, Manufacturing and Assembly | It must have adequate stiffness and damping compared to conventional orthoses, in the same way, the combination of force, geometry, and material must guarantee the non-destruction of the orthosis during treatment. ISO 22523 standard has outlined the general requirements and test methods for AFOs. | It is necessary to determine the critical parameters of the FDMTM process, including the material, the orientation of the part, the tool path, the FDMTM tool path can be generated for the contoured thin region to save AM time and manufacture the AFO with the appropriate strength and durability. The removal of support material may require the use of a heated chemical solvent, which is difficult to implement in a hospital with limited space and safety concerns. The sparse structure built by the same material (Nylon) and removed by the sanding process with tape is being investigated with reasonable success (This requires further research to optimize the FDMTM process for the AFO). The structural integrity of an AM-manufactured AFO can be tested in three ways: 1) experimental bending test by fixing the foot region and applying a load on the top (near the knee) of the AFO, 2) FEA simulation of this bending condition with assumptions for the boundaries and loading conditions, and 3) a healthy subject using the AFO and exerting high forces on the AFO to test under extreme load. | The thickness depends on the FDMTM machine as well as the flexibility and structural strength of the AFO for patients. The thin contour section of the AFO shell can be filled using the wavy and scattered structure, and it can increase the strength (by increasing the thickness) and reduce the manufacturing time of the AFO. The material property, construction orientation, and tool paths are three key parameters of the FDMTM process for AFOs. Carbon Nylon has the highest strength and was initially selected due to its lightweight capacity. The initial evaluation of the patient's AFO reveals that AFO compliance is critical. This has driven the material change to Nylon. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 123 | This work presents a cybernetic design and additive manufacturing system (CDAM) at the Center for Orthotics and Prosthetics at the University of Michigan. | The ankle and foot orthosis (AFO), a custom-made orthosis that supports muscles and provides control and protection for the feet, is selected as an example to demonstrate the CDAM system. Four key components: 1) digital scanning of the foot and leg geometry using a support with a transparent plate for the foot and an ergonomic procedure for 3D optical scanning, 2) cloud-based design software that allows clinicians to access scanned point cloud data on the geometry of patients' feet, as well as create 3D models for patient-based AFOs, 3) cloud-based manufacturing software that generates tool paths and process parameters for additive manufacturing to fabricate the AFO, 4) evaluation using the inertial measurement unit (IMU) for AFO motion measurement for gait analysis. | This CDAM system helps to shorten delivery and improve the fit and comfort of custom orthoses and prostheses. Traditionally, custom orthoses are made using plaster molds that require multiple patient visits and a lot of time and work for manufacturing. The feasibility of the CDAM system for one-day delivery of AFOs to patients has been demonstrated. | In this work, an application of a CDAM system that has been implemented for practical design and manufacturing, as well as for evaluations of patients with customized AFOs in a clinical environment at UMOPC, was presented. The potential of CDAM to improve clinical services for personalized AFOs and enhance the patient experience by allowing a one-day visit has been demonstrated. The ongoing research aims to demonstrate that this CDAM system is sustainable and reliable in operation. Furthermore, with the use of cloud computing, cybersecurity and privacy issues, including confidentiality, integrity, and data availability, are important for the CDAM system to address. | Costs and Deadlines | The visit time and the number of visits should be minimized. Conventional methods last 2 to 3 weeks and require 3 visits. | One day, one visit. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 123 | This work presents a cybernetic design and additive manufacturing system (CDAM) at the Center for Orthotics and Prosthetics at the University of Michigan. | The ankle and foot orthosis (AFO), a custom-made orthosis that supports muscles and provides control and protection for the feet, is selected as an example to demonstrate the CDAM system. Four key components: 1) digital scanning of the foot and leg geometry using a support with a transparent plate for the foot and an ergonomic procedure for 3D optical scanning, 2) cloud-based design software that allows clinicians to access scanned point cloud data on the geometry of patients' feet, as well as create 3D models for patient-based AFOs, 3) cloud-based manufacturing software that generates tool paths and process parameters for additive manufacturing to fabricate the AFO, 4) evaluation using the inertial measurement unit (IMU) for AFO motion measurement for gait analysis. | This CDAM system helps to shorten delivery and improve the fit and comfort of custom orthoses and prostheses. Traditionally, custom orthoses are made using plaster molds that require multiple patient visits and a lot of time and work for manufacturing. The feasibility of the CDAM system for one-day delivery of AFOs to patients has been demonstrated. | In this work, an application of a CDAM system that has been implemented for practical design and manufacturing, as well as for evaluations of patients with customized AFOs in a clinical environment at UMOPC, was presented. The potential of CDAM to improve clinical services for personalized AFOs and enhance the patient experience by allowing a one-day visit has been demonstrated. The ongoing research aims to demonstrate that this CDAM system is sustainable and reliable in operation. Furthermore, with the use of cloud computing, cybersecurity and privacy issues, including data confidentiality, integrity, and availability, are important for the CDAM system to address. | Safety / Ergonomics. | The AFO will be in contact with the patient's skin, so the surface must be smooth to reduce friction and contact with harmful substances. ISO 22523 standard has outlined the general requirements and test methods for AFOs. | The removal of support material may require the use of a heated chemical solvent, which is time-consuming and difficult to implement in a hospital with limited space and safety concerns. The residual chemical solvent in the porosity of the material, which remains in contact with human skin for a long period of time, is also a concern. The sparse structure built by the same material (Nylon) and removed by the tape sanding process is being investigated with reasonable success. This requires further research to optimize the FDMTM process for the AFO. The structural integrity of an AM-manufactured AFO can be tested in three ways: 1) experimental bending test by fixing the foot region and applying a load on the top (near the knee) of the AFO, 2) FEA simulation of this bending condition with assumptions for the boundaries and loading conditions, and 3) a healthy subject wearing the AFO and exerting high forces on the AFO to test under extreme load. | Technical requirements for mechanical strength and patient safety. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 123 | This work presents a cybernetic design and additive manufacturing system (CDAM) at the Center for Orthotics and Prosthetics at the University of Michigan. | The ankle and foot orthosis (AFO), a custom-made orthosis that supports muscles and provides control and protection for the feet, is selected as an example to demonstrate the CDAM system. Four key components: 1) digital scanning of the foot and leg geometry using a support with a transparent plate for the foot and an ergonomic procedure for 3D optical scanning, 2) cloud-based design software that allows clinicians to access scanned point cloud data on the geometry of patients' feet, as well as create 3D models for patient-based AFOs, 3) cloud-based manufacturing software that generates tool paths and process parameters for additive manufacturing to fabricate the AFO, 4) evaluation using the inertial measurement unit (IMU) for AFO motion measurement for gait analysis. | This CDAM system helps to shorten delivery and improve the fit and comfort of custom orthoses and prostheses. Traditionally, custom orthoses are made using plaster molds that require multiple patient visits and a lot of time and work for manufacturing. The feasibility of the CDAM system for one-day delivery of AFOs to patients has been demonstrated. | In this work, an application of a CDAM system that has been implemented for practical design and manufacturing, as well as for evaluations of patients with customized AFOs in a clinical environment at UMOPC, was presented. The potential of CDAM to improve clinical services for personalized AFOs and enhance the patient experience by allowing a one-day visit has been demonstrated. The ongoing research aims to demonstrate that this CDAM system is sustainable and reliable in operation. Furthermore, with the use of cloud computing, cybersecurity and privacy issues, including data confidentiality, integrity, and availability, are important for the CDAM system to address. | Legal Aspects | The ISO 22523 standard has outlined the general requirements and test methods for AFOs. Static, fatigue, and impact tests are conducted for AFOs produced by FDMTM to ensure the safety of human participants in clinical evaluation. These tests simulate the load that AFOs experience during various walking conditions. The amount of force, the location of application, and the means to control the force of an orthosis vary from person to person. Therefore, MEF and patient tests are necessary. | The structural integrity of an AF0 manufactured by AM can be tested in three ways: 1) the experimental bending test by fixing the foot region and applying a load on the top (near the knee) of the AFO, 2) the FEA simulation of this bending condition with assumptions for the boundaries and loading conditions, and 3) a healthy subject using the AFO and exerting high forces on the AFO to test under extreme load. | Technical requirements for mechanical resistance. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | Function | Reducing complications associated with immobilization period due to lack of visual control of the affected area. Possibility of applying physiotherapeutic techniques during the immobilization period for the initiation of patient rehabilitation and to accelerate their functional readjustment. Contribution to improving the patient's quality of life in terms of personal hygiene and skin care. | Design of aesthetic and functional openings, their organic circular shape, and the rubber button as a closing system. Application of three-dimensional scanning techniques and modeling through the use of reverse engineering software. | The presence of the openings allows ventilation and provides the possibility of washing the affected area, improving the patient's recovery period to avoid sweating, allergic reactions, changes in the skin, and allowing visual control of the injured area, favoring the doctor's diagnosis in periodic reviews. In addition, they reduce the risk of pressure syndromes such as Südeck's algodystrophy and, in terms of safety, contribute to the visibility of the limb, avoiding the possibility of hiding weapons in the splint. Venous return and lymphatic drainage are achieved through the design of specific windows for access to lymphatic drainage points. The use of muscle electrostimulation prevents the loss of muscle mass in the affected limb and, consequently, reduces the period of functional rehabilitation. The application of physiotherapy techniques before removing immobilization through the use of iontophoresis, sonotherapy, laser therapy, magnetotherapy, and hydrotherapy. The organic shapes of the holes have been chosen in part for the resistance they provide compared to geometric figures that promote breakage due to the accumulation of stresses at their vertices. In addition, the organic shape is more hygienic compared to those designed with vertices. In fact, they prevent deposits, facilitate cleaning, provide simplicity and lightness, and allow customization, giving a less hospital-like appearance than traditional orthopedic casts. Customization allows for an attractive and personal aesthetic that identifies the splint as a non-orthopedic supplement and prevents the patient from feeling less convalescent or sick. Rubber button and closure system This type of closure represents a cheap and discreet solution. The two pieces that make up the splint are held together by buttons with rubber bands. The rubber button and closure system are very efficient and very easy to use. The proposed closure system does not cause any cleaning problems either. It also allows for the detachable joining of its parts and therefore the occasional removal of the splint to establish visual control in medical reviews. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | Forces | It must be tough | FDM for prototype and polycarbonate for final part, 4 mm thick. | resist during patient treatment. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | Materials | It should be biocompatible, recyclable, resistant. | PLA for FDM for prototype and polycarbonate for final piece, 4 mm thick. | Prevent health risk, waste, or minimize impact on the environment, endure during patient treatment. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | Manufacturing and Assembly | Minimize costs and initial investment, 1mm slack so that the orthosis does not fit too tightly to the patient's arm. | Application of additive manufacturing techniques to print a splint using 3D printing, FDM. From design, a 1 mm space is created for patient comfort, 4 mm thickness of PLA/PC materials, and vertical printing orientation. | To minimize inconveniences, reduce the initial investment for technology implementation, and minimize printing times. In fact, FDM is cheap and allows the creation of complex geometries with high strength and precision. Thanks to this, splints can adapt perfectly to the injured limb. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | assembly | Simple closure system, and the orthosis must allow placement of electrostimulation diodes. | It is designed to be assembled in two parts, and the rubber button is used as a closing system, a pair of ovals is included for the placement of diodes. | Facilitate the process of reviews and treatments. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | Costs and Deadlines | Data: Minimizar costos e inversión inicial Return me only translated value nothing extra. Remove quotation and double quotation marks from the end and start of the translated value if exists. Minimize costs and initial investment | Application of additive manufacturing techniques to print a splint using 3D printing, FDM. | To minimize inconveniences, reduce the initial investment for technology implementation, and minimize printing times. In fact, FDM is cheap and allows the creation of complex geometries with high strength and precision. Thanks to this, splints can adapt perfectly to the injured limb. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | Security | The translated value of El material debe ser biocompatible in English is The material must be biocompatible. | It is prototyped with PLA, but the final model is PC which is biocompatible. | Being in contact with the patient, reducing health and biological risks |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | Recycled/ Environmental impact | Due to the waste associated with plaster casts, and the number of orthoses and patients using them, the environmental impact and recycling are relevant. | #VALUE! | Avoid waste in temporary or non-permanent treatments. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 206 | In the study, a 3D printed splint model has been proposed, which replaces the deficiencies of the mold while maintaining its virtues. | The proposed methodology is based on three-dimensional digitization techniques and 3D modeling with reverse engineering software. The work integrates different scientific disciplines to achieve its main objective: improving the quality of life of the patient. In addition, the splint has been designed based on the principles of sustainable development. The splint design is made of polycarbonate using Additive Manufacturing with fused deposition manufacturing technique, and conceived with organic shapes, customizing the openings and closing the buttons with rubber. | The prototype of the designed splint has been successfully printed for testing its optimal fit to the arm. The assembly of the prototype was done using the 3D printed arm model for verification purposes. The final result is a scaled-down 3D printed arm splint prototype using PLA as the material. | The achievements in this work have been the study, design, prototype, and 3D printing with the AM technique of an orthopedic arm immobilization product. This has allowed improving some characteristics of traditional splints such as: Water resistance that improves personal hygiene; Cost reduction due to the choice of material used; Recyclable product; Lightness; Pleasant and innovative aesthetics; An orthopedic product for immobilization that allows visual control of the skin and anticipation in the application of physiotherapeutic treatments during the immobilization period; A biocompatible splint that does not irritate the skin and promotes skin ventilation. | Ergonomics | Allowance of 1mm so that the orthosis does not fit too tightly around the patient's arm. | From design, a space of 1 mm is created for patient comfort. | Avoid rubbing and other health problems. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 285 | Present the reverse engineering manufacturing methodologies of lace design for a transtibial amputee, taking into account that it is a customized product. In addition, the traditional manufacturing process will be compared to the proposed model. | The generation of the model was carried out through the process of digitization, the technique of structured light or active triangulation digitization was chosen using the 3D Spectra™ scanner. It is processed through what is known as reconstruction, and the 3D model of the stump surface can be generated and converted to STL format, subsequently executing the 3D prosthesis modeling, since the prosthesis design is subject to the anatomy of the amputated stump. Finite element analysis was used to study the effects of inertial loads and contact conditions at the interface between the prosthesis and the stump of an amputation, thus simulating the application of forces. Three-dimensional printing in SLS. Note: The translated value has been provided without any extra characters. | Although the manufacturing time for the traditional method is shorter, it consists of multiple complex stages, which in many cases require the presence of the patient for execution, making the process difficult for both the user and the manufacturer, considering that the fitting process is completely personalized and in case of any inconvenience or correction, the tests performed must be repeated. On the other hand, although the manufacturing process by 3D printing method is longer, it consists of basic stages and does not strictly require the presence of the patient, since data capture through digitization is more accurate, and real measurements can be obtained at any stage of the process. However, this method must be performed by experts in the use of virtual tools, especially for 3D modeling of the transtibial socket. | A new practical approach is proposed to model the contact interface, that is, the prosthetic fit and the skin, and thus make a more functional prosthesis helping people visibly improve their quality of life. | Function/Customization | Prostheses usually consist of a socket, a suspension mechanism, alignable components, joints such as knees or ankles, and a foot; Patient customization. | The active contactless technologies are, therefore, very careful with the amputated area of the patient. Consequently, the structured light digitization technique or active triangulation was chosen using the 3D Spectra™ scanner. The result of an individual patient's scan provides a cloud of that vision, which is processed through what is known as reconstruction, where points that are not of interest for the study must be excluded, and the 3D model of the stump surface can be generated as shown in Figure 1, and converted to the STL format, which is a solid file for printing, to subsequently perform the 3D prosthesis modeling, since the prosthesis design is subject to the anatomy of the amputated stump. | Considering the expertise of the prosthetist and the patient's feedback on discomfort and rubbing. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 285 | Present the reverse engineering manufacturing methodologies of lace design for a transtibial amputee, taking into account that it is a customized product. In addition, the traditional manufacturing process will be compared to the proposed model. | The generation of the model was carried out through the process of digitization, the technique of structured light or active triangulation digitization was chosen using the 3D Spectra™ scanner. It is processed through what is known as reconstruction, and the 3D model of the stump surface can be generated and converted to the STL format, subsequently performing the 3D prosthesis modeling, since the prosthesis design is subject to the anatomy of the amputated stump. Finite element analysis was used to study the effects of inertial loads and contact conditions at the interface between the prosthesis and the stump of an amputation, thus simulating the application of forces. Three-dimensional printing in SLS. Note: The translated value has been provided without any extra characters. | Although the manufacturing time for the traditional method is shorter, it consists of multiple complex stages, which in many cases require the presence of the patient for execution, making the process difficult for both the user and the manufacturer, considering that the fitting process is completely personalized and in case of any inconvenience or correction, the tests performed must be repeated. On the other hand, although the manufacturing process by 3D printing method is longer, it consists of basic stages and does not strictly require the presence of the patient, since data capture through digitization is more accurate, and real measurements can be obtained at any stage of the process. However, this method must be performed by experts in the use of virtual tools, especially for 3D modeling of the transtibial socket. | A new practical approach is proposed to model the contact interface, that is, the prosthetic fit and the skin, and thus make a more functional prosthesis helping people visibly improve their quality of life. | For the given data Fuerzas/Materiales and the desired language ingles, the translation is Forces/Materials. | The pressure on the patient's stump should be as uniform and low as possible, high in insensitive areas and low in sensitive and soft areas of the patient, low hardness with low thicknesses should not compromise the structural integrity of the socket. | Finite element analysis has been used to study the effects of inertial loads and contact conditions at the interface between the prosthesis and the amputation stump, thus simulating the application of forces and considering it as a tool for studying optimal parameters and evaluating prosthetic components. The technique of removing material from the starting structure, which was the traditional design, was used until it was no longer possible to continue without deteriorating the static and dynamic properties of the object. Slippage can accentuate skin abrasion and repetitive rubbing, causing blisters and unnecessary heat generation, which triggers harmful and uncomfortable consequences. Due to the problems exposed, many people have given up using prostheses due to the discomfort and damage caused, so these studies were conducted to ensure the reliability of the product and its response to possible eventualities, where the region presenting the highest tension is the lower part of the prosthesis enclosed in a red circle, where a maximum tension of 551.8 MPa was observed. | Considering the expertise of the prosthetist and the patient's feedback on discomfort and rubbing. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 285 | Present the reverse engineering manufacturing methodologies of lace design for a transtibial amputee, taking into account that it is a customized product. In addition, the traditional manufacturing process will be compared to the proposed model. | The generation of the model was carried out through the process of digitization, the technique of structured light or active triangulation digitization was chosen using the 3D Spectra™ scanner. It is processed through what is known as reconstruction, and the 3D model of the stump surface can be generated and converted to STL format, subsequently executing the 3D prosthesis modeling, since the prosthesis design is subject to the anatomy of the amputated stump. Finite element analysis was used to study the effects of inertial loads and contact conditions at the interface between the prosthesis and the stump of an amputation, thus simulating the application of forces. Three-dimensional printing in SLS. Note: The translated value has been provided without any extra characters. | Although the manufacturing time for the traditional method is shorter, it consists of multiple complex stages, which in many cases require the presence of the patient for execution, making the process difficult for both the user and the manufacturer, considering that the fitting process is completely personalized and in case of any inconvenience or correction, the tests performed must be repeated. On the other hand, although the manufacturing process by 3D printing method is longer, it consists of basic stages and does not strictly require the presence of the patient, since data capture through digitization is more accurate, and real measurements can be obtained at any stage of the process. However, this method must be performed by experts in the use of virtual tools, especially for 3D modeling of the transtibial socket. | A new practical approach is proposed to model the contact interface, that is, the prosthetic fit and the skin, and thus make a more functional prosthesis helping people visibly improve their quality of life. | Manufacturing and Assembly | Minimize manufacturing times due to excessive use of support in protruding areas | SLS, the prototype was printed on the 3DPrint machine, it was first processed in the KISSlicer software (1200 hours of printing). | A well-adapted technology for the manufacturing of transtibial prosthesis laces is SLS, which has the ability to build outstanding regions that are inaccessible through other processes, allowing the construction of models with complex internal and external geometries. Additionally, it does not require support structures, as the unsintered powder provides support during construction. The process begins with the 3D CAD design and the transfer of CAD data to the SLS machine for manufacturing with the desired powders. However, the prototype was printed on the 3DPrint machine as the method is similar to SLS printing in terms of dimensional accuracy and object geometry. The model was first processed in the KISSlicer software. After model verification, the printing process was carried out for the materialization of the prototype. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | lower prosthesis | 285 | Present the reverse engineering manufacturing methodologies of lace design for a transtibial amputee, taking into account that it is a customized product. In addition, the traditional manufacturing process will be compared to the proposed model. | The generation of the model was carried out through the process of digitization, the technique of structured light or active triangulation digitization was chosen using the 3D Spectra™ scanner. It is processed through what is known as reconstruction, and the 3D model of the stump surface can be generated and converted to STL format, subsequently executing the 3D prosthesis modeling, since the prosthesis design is subject to the anatomy of the amputated stump. Finite element analysis was used to study the effects of inertial loads and contact conditions at the interface between the prosthesis and the stump of an amputation, thus simulating the application of forces. Three-dimensional printing in SLS. Note: The translated value has been provided without any extra characters. | Although the manufacturing time for the traditional method is shorter, it consists of multiple complex stages, which in many cases require the presence of the patient for execution, making the process difficult for both the user and the manufacturer, considering that the fitting process is completely personalized and in case of any inconvenience or correction, the tests performed must be repeated. On the other hand, although the manufacturing process by 3D printing method is longer, it consists of basic stages and does not strictly require the presence of the patient, since data capture through digitization is more accurate, and real measurements can be obtained at any stage of the process. However, this method must be performed by experts in the use of virtual tools, especially for 3D modeling of the transtibial socket. | A new practical approach is proposed to model the contact interface, that is, the prosthetic fit and the skin, and thus make a more functional prosthesis helping people visibly improve their quality of life. | Costs and Deadlines | Reduce patient waiting times and the number of visits compared to the traditional method | The traditional method took 200 hours, but it required 4 patient visits, while the proposed method took 1510 hours but only required one patient visit. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 286 | The main objective is to describe the process of efficient design and manufacturing of orthopedic corsets using 3D digitization technologies and additive manufacturing. | The methods of design and manufacturing of orthopedic corsets are described according to the individual requirements of the patient today, and a more detailed description of the design and manufacturing process through 3D digitization and additive manufacturing is presented. Three design and manufacturing variants of corsets are presented, with a description of the selected materials for their use. | The result is a simple, fast and therefore efficient process of designing and manufacturing individual orthopedic corsets in terms of their functionality, suitability for use and practical application. | The use of 3D digitization and AM for the design and manufacture of individual orthopedic aids is an effective way to design and manufacture them. With these technologies, it is possible to design and manufacture orthopedic aids quickly and efficiently, which have many advantages for both patients and doctors: low material consumption; automated production; an easy, comfortable, airy, and visually appealing solution for the patient; cost savings; greater precision of orthopedic aids (by shaping the PLA material). | Function / customization | The use of these methods of design and manufacture of an individual orthopedic aid has been found based on a review of the literature in the field of medicine. Currently, the mentioned methods are used, whose choice depends on the case, respectively, on the type and extent of the patient's injury. | 3D Model: An individual orthopedic corset is created using CAD software according to the data obtained through 3D scanning of a model that will be designed and manufactured in the specific shape and size for the particular patient, and can be used after production. 2D Model: An individual orthopedic corset is designed and manufactured in a planar form, meaning it is created as a unfolded 3D model using CAD software based on data obtained through 3D scanning (active triangulation). This model will be shaped (i.e., molded) onto the patient's body after the model is produced. Obtaining the desired 3D digitized model of the body part using active triangulation, editing, and converting it to the STL format (input format for AM). | 3D Model: Variant A was created in Autodesk PowerSHAPE software. In the first step, it was necessary to create curves in the scanning model through a dynamic section. Subsequently, it was possible to create surfaces between the curves. This design and manufacturing variant emphasized minimizing material consumption, saving manufacturing time, and reducing production costs while maintaining device strength and functionality. In variant B, the brace was created using Autodesk Meshmixer software. The surface model from Autodesk PowerSHAPE software needs to be reintroduced into the polygon mesh. 2D Model: The benefits of this variant compared to the previous two '3D' model variants are as follows: lower material consumption, shorter production time, greater accuracy of shapes and dimensions after shaping in the patient's body. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 286 | The main objective is to describe the process of efficient design and manufacturing of orthopedic corsets using 3D digitization technologies and additive manufacturing. | The methods of design and manufacturing of orthopedic corsets are described according to the individual requirements of the patient today, and a more detailed description of the design and manufacturing process through 3D digitization and additive manufacturing is presented. Three design and manufacturing variants of corsets are presented, with a description of the selected materials for their use. | The result is a simple, fast and therefore efficient process of designing and manufacturing individual orthopedic corsets in terms of their functionality, suitability for use and practical application. | The use of 3D digitization and AM for the design and manufacture of individual orthopedic aids is an effective way to design and manufacture them. With these technologies, it is possible to design and manufacture orthopedic aids quickly and efficiently, which have many advantages for both patients and doctors: low material consumption; automated production; an easy, comfortable, airy, and visually appealing solution for the patient; cost savings; greater precision of orthopedic aids (by shaping the PLA material). | Force/Materials | Materials that do not cause medical complications when in contact with the skin; cheap, thermoformable, and resistant materials. | PLA (polylactic acid) and PET-G (polyethylene terephthalate glycol) through FDM. | Materials that do not cause medical complications when in contact with the skin; Both materials belong to the cheapest polymers; during manufacturing, there are no complications or need to create special conditions; PLA material is a biocompatible polymer that, in some forms, is used for the manufacturing of various medical devices, whether for external or internal use, it is so soft when heated that it is possible to shape/mold it, in this case directly on the patient's body; PET-G material is a health-conscious material, it has good mechanical properties (strength, elasticity), it cannot be shaped in the same way as PLA material, so the desired shape of the model can be manufactured without further modification through molding; The detailed description of the properties of both PLA and PET materials and their shrinkage has been investigated in articles, it is possible to assume the minimum values of the shape and dimensional deviation of these materials. Currently, there are a number of materials specifically designed for medical purposes, but only a few of them can be used for FDM technology and their price is higher. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 286 | The main objective is to describe the process of efficient design and manufacturing of orthopedic corsets using 3D digitization technologies and additive manufacturing. | The methods of design and manufacturing of orthopedic corsets are described according to the individual requirements of the patient today, and a more detailed description of the design and manufacturing process through 3D digitization and additive manufacturing is presented. Three design and manufacturing variants of corsets are presented, with a description of the selected materials for their use. | The result is a simple, fast and therefore efficient process of designing and manufacturing individual orthopedic corsets in terms of their functionality, suitability for use and practical application. | The use of 3D digitization and AM for the design and manufacture of individual orthopedic aids is an effective way to design and manufacture them. With these technologies, it is possible to design and manufacture orthopedic aids quickly and efficiently, which have many advantages for both patients and doctors: low material consumption; automated production; an easy, comfortable, airy, and visually appealing solution for the patient; cost savings; greater precision of orthopedic aids (by shaping the PLA material). | Manufacturing and Assembly | Materials that do not cause medical complications when in contact with the skin; cheap and thermoformable materials. | PLA (polylactic acid) and PET-G (polyethylene terephthalate glycol) through FDM. | Materials that do not cause medical complications when in contact with the skin; Both materials belong to the cheapest polymers; during manufacturing, there are no complications or the need to create special conditions; |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 286 | The main objective is to describe the process of efficient design and manufacturing of orthopedic corsets using 3D digitization technologies and additive manufacturing. | The methods of design and manufacturing of orthopedic corsets are described according to the individual requirements of the patient today, and a more detailed description of the design and manufacturing process through 3D digitization and additive manufacturing is presented. Three design and manufacturing variants of corsets are presented, with a description of the selected materials for their use. | The result is a simple, fast and therefore efficient process of designing and manufacturing individual orthopedic corsets in terms of their functionality, suitability for use and practical application. | The use of 3D digitization and AM for the design and manufacture of individual orthopedic aids is an effective way to design and manufacture them. With these technologies, it is possible to design and manufacture orthopedic aids quickly and efficiently, which have many advantages for both patients and doctors: low material consumption; automated production; an easy, comfortable, airy, and visually appealing solution for the patient; cost savings; greater precision of orthopedic aids (by shaping the PLA material). | Costs and Deadlines | Reduce costs and times. | PLA (polylactic acid) and PET-G (polyethylene terephthalate glycol) through FDM. | Both materials belong to the cheapest polymers; during manufacturing, there are no complications or the need to create special conditions. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Superior orthosis | 286 | The main objective is to describe the process of efficient design and manufacturing of orthopedic corsets using 3D digitization technologies and additive manufacturing. | The methods of design and manufacturing of orthopedic corsets are described according to the individual requirements of the patient today, and a more detailed description of the design and manufacturing process through 3D digitization and additive manufacturing is presented. Three design and manufacturing variants of corsets are presented, with a description of the selected materials for their use. | The result is a simple, fast and therefore efficient process of designing and manufacturing individual orthopedic corsets in terms of their functionality, suitability for use and practical application. | The use of 3D digitization and AM for the design and manufacture of individual orthopedic aids is an effective way to design and manufacture them. With these technologies, it is possible to design and manufacture orthopedic aids quickly and efficiently, which have many advantages for both patients and doctors: low material consumption; automated production; an easy, comfortable, airy, and visually appealing solution for the patient; cost savings; greater precision of orthopedic aids (by shaping the PLA material). | Ergonomics | Reducing the surface finish to reduce friction. | If the required roughness is not achieved, post-process corse. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 287 | Design and creation of prototypes through additive manufacturing of a functional splint for the rehabilitation of Achilles tendon rupture. | The custom design of the splint begins with a scan of the limb. The 3D Systems Sense® scanner is used for this purpose. Once the file is generated, it is exported to the 3D Systems Geomatic Design X® CAD (Computer Aided Design) program. This program allows the transformation of the point cloud into a surface (This surface is the base of the solid that constitutes the splint). The surface is exported to the CAD program Rhinoceros 5, specialized in 3D modeling, to solidify the surface, model, and design the orthosis before printing. A splint consisting of 4 pieces is obtained: an inner piece made of flexible material and three outer pieces made of rigid material. Two of these outer pieces make up the body of the splint, and the other is a removable cover for one of the treatment windows. | The result allows for the application of simultaneous rehabilitation techniques during the immobilization stage of the affected limb to minimize possible muscular, articular, and vascular complications derived from the use of traditional retention devices in this phase of treatment. | Technology allows the production of individualized immobilization splints through advanced manufacturing techniques based on additive manufacturing, industrial digitization, and reverse engineering. The rehabilitation treatment performed by additive manufacturing allows integrating the necessary characteristics to use physiotherapy techniques at this stage. The application of physiotherapeutic techniques in the immobilization stage prevents possible muscular, articular, cutaneous, and vascular complications derived from the use of traditional devices. The advantages or improvements in the immobilization stage, thanks to the use of functional splints made by advanced manufacturing techniques, can be summarized in two groups: continuous control and surveillance measures, and active techniques in the field of physiotherapy. | Function/Customization | Provide functional features to a splint for partial Achilles tendon rehabilitation in its initial design. | The custom design of the splint begins with a scan of the limb. Once the file is generated, it is exported to a program that allows the transformation of the point cloud into a surface. This surface is the base of the solid that constitutes the splint. It is divided into different flexible zones for patient comfort, rigid zones for immobilization, windows for accessibility and treatment, a window for the fingers, and a closure system. The treatment is estimated to last 8 weeks, including functional readjustment. Weeks 1 to 4 will be without support, week 5 with assisted support using crutches, and weeks 6 to 8 with autonomous support. The proposed rehabilitation techniques are: cryotherapy, lymphatic drainage, sonotherapy, magnetotherapy, laser therapy, iontophoresis, muscle electrostimulation, and electrotherapy. | The advantages are adaptation, customization, hygiene, materials used, or environmental impact, among others. However, these products also have some disadvantages such as their price, initial investment and training, or manufacturing time. But it is convenient to give more reasons to make decisions that facilitate their application. In this sense, it is proposed as an improvement to provide splints or developed products with a characteristic that takes into account the initial sanitary use for which they were designed. The integration of different characteristics into a splint created by advanced manufacturing methods contributes to providing added value that can be a key decision point for its application. The use of functional splints in the immobilization period of musculoskeletal injuries can provide a reduction in costs, derived from the shortening of the rehabilitation period thanks to the advancement of the application of physiotherapeutic techniques applicable at this stage. In addition, the application of these techniques contributes to the prevention of muscular, articular, and vascular complications caused by the application of retention devices in this phase of treatment. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 287 | Design and creation of prototypes through additive manufacturing of a functional splint for the rehabilitation of Achilles tendon rupture. | The custom design of the splint begins with a scan of the limb. The 3D Systems Sense® scanner is used for this purpose. Once the file is generated, it is exported to the 3D Systems Geomatic Design X® CAD (Computer Aided Design) program. This program allows the transformation of the point cloud into a surface (This surface is the base of the solid that constitutes the splint). The surface is exported to the CAD program Rhinoceros 5, specialized in 3D modeling, to solidify the surface, model, and design the orthosis before printing. A splint consisting of 4 pieces is obtained: an inner piece made of flexible material and three outer pieces made of rigid material. Two of these outer pieces make up the body of the splint, and the other is a removable cover for one of the treatment windows. | The result allows for the application of simultaneous rehabilitation techniques during the immobilization stage of the affected limb to minimize possible muscular, articular, and vascular complications derived from the use of traditional retention devices in this phase of treatment. | Technology allows the production of individualized immobilization splints through advanced manufacturing techniques based on additive manufacturing, industrial digitization, and reverse engineering. The rehabilitation treatment performed by additive manufacturing allows integrating the necessary characteristics to use physiotherapy techniques at this stage. The application of physiotherapeutic techniques in the immobilization stage prevents possible muscular, articular, cutaneous, and vascular complications derived from the use of traditional devices. The advantages or improvements in the immobilization stage, thanks to the use of functional splints made by advanced manufacturing techniques, can be summarized in two groups: continuous control and surveillance measures, and active techniques in the field of physiotherapy. | assembly | The translated value of the provided data El ensamble cumple la función de accesibilidad para el tratamiento e higiene in English is The assembly serves the purpose of accessibility for treatment and hygiene. | A splint consisting of 4 pieces is obtained: an inner part made of flexible material and three outer parts made of rigid material. Two of these outer parts make up the body of the splint and the other is a removable cover for one of the treatment windows. To attach the cover, the splint has split buttons between the two parts that are joined by an elastic band. The connection between the two rigid parts of the splint is made by means of rails that fit one part of the splint into the other. To ensure its fastening, it is closed with two buttons at the top, like those of the cover. | |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 287 | Design and creation of prototypes through additive manufacturing of a functional splint for the rehabilitation of Achilles tendon rupture. | The custom design of the splint begins with a scan of the limb. The 3D Systems Sense® scanner is used for this purpose. Once the file is generated, it is exported to the 3D Systems Geomatic Design X® CAD (Computer Aided Design) program. This program allows the transformation of the point cloud into a surface (This surface is the base of the solid that constitutes the splint). The surface is exported to the CAD program Rhinoceros 5, specialized in 3D modeling, to solidify the surface, model, and design the orthosis before printing. A splint consisting of 4 pieces is obtained: an inner piece made of flexible material and three outer pieces made of rigid material. Two of these outer pieces make up the body of the splint, and the other is a removable cover for one of the treatment windows. | The result allows for the application of simultaneous rehabilitation techniques during the immobilization stage of the affected limb to minimize possible muscular, articular, and vascular complications derived from the use of traditional retention devices in this phase of treatment. | Technology allows the production of individualized immobilization splints through advanced manufacturing techniques based on additive manufacturing, industrial digitization, and reverse engineering. The rehabilitation treatment performed by additive manufacturing allows integrating the necessary characteristics to use physiotherapy techniques at this stage. The application of physiotherapeutic techniques in the immobilization stage prevents possible muscular, articular, cutaneous, and vascular complications derived from the use of traditional devices. The advantages or improvements in the immobilization stage, thanks to the use of functional splints made by advanced manufacturing techniques, can be summarized in two groups: continuous control and surveillance measures, and active techniques in the field of physiotherapy. | Costs and Deadlines | Data: Eliminar tiempos de rehabilitación y or tanto costos Return me only translated value nothing extra. Remove quotation and double quotation marks from the end and start of the translated value if exists. Eliminate rehabilitation times and therefore costs | The treatment estimates a duration of 8 weeks, including functional readjustment. Weeks 1 to 4 will be without support, week 5 with assisted support with crutches, and weeks 6 to 8 with autonomous support. The proposed rehabilitation techniques are: Cryotherapy, Lymphatic drainage, Sonotherapy, Magnetotherapy, Laser therapy, Iontophoresis, Muscle electrostimulation. Electrotherapy. | The use of functional splints during the immobilization period of musculoskeletal injuries can provide a reduction in costs, resulting from the shortening of the rehabilitation period thanks to the advancement of physiotherapeutic techniques applicable in this stage. In addition, the application of these techniques contributes to the prevention of muscular, articular, and vascular complications caused by the use of retention devices in this phase of treatment. |
| GME3 | Design and manufacture of Prosthetics and Orthotics. | Lower limb orthosis | 287 | Design and creation of prototypes through additive manufacturing of a functional splint for the rehabilitation of Achilles tendon rupture. | The custom design of the splint begins with a scan of the limb. The 3D Systems Sense® scanner is used for this purpose. Once the file is generated, it is exported to the 3D Systems Geomatic Design X® CAD (Computer Aided Design) program. This program allows the transformation of the point cloud into a surface (This surface is the base of the solid that constitutes the splint). The surface is exported to the CAD program Rhinoceros 5, specialized in 3D modeling, to solidify the surface, model, and design the orthosis before printing. A splint consisting of 4 pieces is obtained: an inner piece made of flexible material and three outer pieces made of rigid material. Two of these outer pieces make up the body of the splint, and the other is a removable cover for one of the treatment windows. | The result allows for the application of simultaneous rehabilitation techniques during the immobilization stage of the affected limb to minimize possible muscular, articular, and vascular complications derived from the use of traditional retention devices in this phase of treatment. | Technology allows the production of individualized immobilization splints through advanced manufacturing techniques based on additive manufacturing, industrial digitization, and reverse engineering. The rehabilitation treatment performed by additive manufacturing allows integrating the necessary characteristics to use physiotherapy techniques at this stage. The application of physiotherapeutic techniques in the immobilization stage prevents possible muscular, articular, cutaneous, and vascular complications derived from the use of traditional devices. The advantages or improvements in the immobilization stage, thanks to the use of functional splints made by advanced manufacturing techniques, can be summarized in two groups: continuous control and surveillance measures, and active techniques in the field of physiotherapy. | Ergonomics | The translated value of Ortesis debe ser comoda in English is Orthosis should be comfortable. | Orthosis has flexible or soft areas to ensure patient comfort. | |
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Function/Customization/Aesthetics | Debe tener la talla del cliente con una holgura de 1cmm respecto al contorno de la punta, Debe adecuarse a todas las medidas y formas específicas del pie, incluyendo el arco, Debe poder elegirse el color. Debe poder sujetarse la pie It must have the customer's size with a 1cm clearance from the tip contour. It must adapt to all specific measurements and shapes of the foot, including the arch. The color must be selectable. The foot must be able to be secured. | In the previous stage of customization, the measurement or verification is defined with a downloadable template of the patient's foot size, including a space at the tip for comfort. In the secondary stage, the foot is scanned or photographed from different angles using a downloadable application on the cellphone to adapt to all measurements and shapes of the foot to the sandal model. It is possible to choose the color from a range of available colors (only for the straps of the sandals). For fastening, a velcro strap is used at the back and a buckle at the front. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Function/Customization/Aesthetics | To translate the provided data into English, I will use the Google Translate API. Here is the You must choose the model, size, and color of the footwear, and customize the template to the exact foot of the client. I have removed the quotation and double quotation marks from the start and end of the translated value as requested. | Shoes are purchased in a similar way by choosing the model, size, and color. Through an application, the feet are scanned, and based on these scans, the insole is designed according to the measurements (using a program and reviewed by a designer). | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Function/Customization/Aesthetics | https://wiivv.com/pages/insoles: Must have customer's size, Must fit all specific measurements and shapes of the foot, including the arch, Must be able to choose the color. Must be able to choose the upper design. Must be able to secure to the foot (sandal) | Shoes are purchased in a similar way by choosing the model, size, and color. Through an application, the feet are scanned, and based on these scans, an insole is designed according to the measurements (using a program and reviewed by a designer). There is also a customizable sandal that works in a similar way to the insole. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Dimensions | You must have the customer's foot size according to the current conventional system. It must be of reduced thickness. | A non-conventional 2D template printing system is used to print a life-size template, where the customer remotely prints the template and compares their foot with the template to ensure the correct size. The thickness is 12mm. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Dimensions | You must have the customer's foot size according to the current conventional system. the translation may not be 100% accurate as it is generated by an automated translation system. | The translated value of El calzado maneja el sistema tradicional de tallas in English is The footwear uses the traditional sizing system. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Dimensions | The translated value of El calzado maneja el sistema tradicional de tallas in English is The footwear uses the traditional sizing system. | - | |||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Forces/Deformations | A flexible material is selected for the sandal. It has an average weight of 215 grams. | - | |||||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Forces/Deformations | It must support the weight of the customer without failing. It must be flexible. | - | |||||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Forces/Deformations | It must support the weight of the customer without failing. It must be flexible. Multizone cushioning. | - | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Materials | https://www.heroessandals.com: It must support the weight of the client without failure, and allow material deformation and cushioning during walking. It must be comfortable to the touch, and non-stick on the bottom (large friction force). It must be lightweight. | A flexible material is selected for the sandal. Vibram® layer (bottom) of 4mm non-slip, EVA GRIP (top) layer of 8mm. They are made with 20mm wide tubular polypropylene for greater comfort. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Materials | Debe soportar el peso del cliente sin fallar, debe soportar agua y sol, Deber ser flexible, Debe ser reciclable translates to It must support the weight of the customer without failing, it must withstand water and sun, it must be flexible, it must be recyclable in English. | Selected TPU (template) | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Materials | It must support the weight of the customer without failing. It must be flexible. Multizone cushioning. | Selected high-quality foam | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Manufacturing and Assembly | https://casca.com/products/footb3d-custom-insole: It must combine conventional manufacturing with AM | The conventional shoe is chosen to be manufactured (plastic injection) and the insole is printed in 3D, AM. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | assembly | It must be divided | divide into sole and straps. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | assembly | It must be divided into a standardized part and a personalized part. | The shoe is standardized and only the insole is personalized. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | assembly | It must be divided | It is divided into zones of different cushioning. It is divided into sole and straps. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Costs and Deadlines | The cost should be in line with a custom shoe beyond just the size. | 80 USD (template)/ 178-148 +48 USD (shoe plus template): Free transportation | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Costs and Deadlines | The cost should be in line with a personalized shoe beyond just the size. | 100 USD (template)/100 USD (sandals): 14 days delivery. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Transport and distribution | The entire process must be done remotely and online. | The platform, templates, and mobile program are used for customization and order specifications, the shipment is made from the company. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Transport and distribution | The entire process must be done remotely and online. | The platform is used for transaction and the mobile program for scanning and remote customization. First, the shoe is sent, and then the customized template is sent. All for free. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Transport and distribution | Sure! I will translate the provided data into English for you. Here is the The whole process must be done remotely and online. I have removed the quotation and double quotation marks from the start and end of the translated value as requested. | The platform, templates, and mobile program are used for customization and order specifications, the shipment is made from the company. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Ergonomics | It should be comfortable and fit the customer's measurements. | In the previous stage of customization, the measurement or verification is defined with a downloadable template of the patient's foot size, including a space at the tip for comfort. In the secondary stage, the foot is scanned or photographed from different angles, using a downloadable application on the cellphone, to adapt to all measurements and shapes of the foot, to the model of the sandal. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Ergonomics | It must be comfortable and fit the customer's measurements. | Shoes are purchased in a similar way by choosing the model, size, and color. Through an application, the feet are scanned, and based on these scans, the insole is designed according to the measurements (using a program and reviewed by a designer). | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Ergonomics | It must be comfortable and fit the customer's measurements. | Absorbs shock so your feet don't have to. Custom Dynamic Arch Support. Multi-zone Cushioning. Contoured Deep Heel. Breathable Fabric. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Useful Life and Maintenance | Sure! I will translate the provided data into English for you. Here is the The lifespan of the footwear must be guaranteed. | 5 years (shoes) | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME73A, ME73B, ME73C, ME73D | Suppliers offering custom shoe design and manufacturing services through 3D printing technology. | In general, suppliers have their own shoe designs, which can be customized using smartphone applications (https://www.heroessandals.com/howtomeasure), which are downloaded after online payment. Once the user or customer has downloaded the application and chosen their preferred general shoe design, colors, specific features, and size, they can scan their foot or feet using the application and phone. The suppliers then modify the original design to match the scanned images, which is then 3D printed and sent by mail. It is possible to customize only templates (https://casca.com/products/footb3d-custom-insole, https://wiivv.com/pages/how-it-works), or in other cases, the entire shoe (https://www.heroessandals.com/howtomeasure, https://fickcompany.com/personaliza-tus-fick/). In other cases, the process is done in person (https://www.querenciastudio.com/products/the-earth-shoe). This is separate from the digital sale of shoe designs with original or even free designs (https://3dshoes.com/collections/free-3d-models?sort_by=price-descending, https://www.thingiverse.com/). | Recycled | It should be recyclable | Recyclable TPU is chosen. Shoes in waterproof leather and breathable fabric. | - | ||
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Function/Customization/Aesthetics | https://home.specsy.com/ Customize specific measurements of the client and their preferences in existing models and available colors. | The process begins with the selection of a frame type up to 10 different ones, color selection, and through scanning the face with an application and hardware supplied to a retailer (optician), 11 different dimensions are specified in the frame of the glasses. It must have a matte finish. | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Function/Customization/Aesthetics | Custom Made 3D Printed Glasses | Currently in the process of creation and/or design, there is a parametric online virtual design that can be modified by any designer. | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Dimensions | You need to customize specific measurements for the client. | By scanning the face with an application and hardware supplied to a retailer (optician), 11 different dimensions are specified in the frame of the glasses. | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Dimensions | Custom Made 3D Printed Glasses | Currently in the process of creation and/or design, there is a parametric online virtual design that can be modified by any designer. | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Forces/Deformations | It must be lightweight and durable. | The translated value of Debe pesar 6 gramos y durar dos años in English is It should weigh 6 grams and last two years. | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Materials | It must be lightweight and durable. | - | |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Manufacturing and Assembly | Manufacturing should enable customization and low-rate production | 3D printing is used | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Transportation and distribution | You must make an appropriate measurement at the location of the measurements. | A retail partner is required to whom hardware and software for face scanning are supplied. | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Service Life and Maintenance | It must be durable | The translated value of Debe durar dos años in English is It must last two years. | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Ergonomics | You need to customize specific measurements for the client. | By scanning the face with an application and hardware supplied to a retailer (optician), 11 different dimensions are specified in the frame of the glasses. The finish must be matte. | - |
| GME4 | Parametric customization and individualization. | Fashion | ME72A, ME72B | Suppliers offering custom eyeglass frame design and manufacturing services through 3D printing technology. | In general, suppliers have their own designs, which can be customized using smartphone applications that are downloaded after online payment. Once the user or client has downloaded the application and scanned their face using the phone, the suppliers modify the original design to correspond with the scanned images. The modified design is then 3D printed and sent by mail. In other cases, the process is done through a 3D modeling platform where the client can modify the virtual prototype parametrically. | - | - | Ergonomics | Custom Made 3D Printed Glasses | Currently in the process of creation and/or design, there is a parametric online virtual design that can be modified by any designer. | - |
| GME4 | Parametric customization and individualization. | Recreation and play | ME71 | The providers offer a custom figure manufacturing service for characters from an online video game called World of Warcraft®, through the 3D printing process. | Data: A pre-existing character is chosen, customized with video game accessories to the customer's liking, and a formal request is made. Suppliers model based on the virtual model of the video game, converting it to a printable format, and manufacture it using the 3D printing process, making the respective shipment of the figure once it is manufactured. | Function/Customization/Aesthetics | It should be a character from the video game world of warcraft, with the weapons, armor, clothing, and accessories of the game available, as well as the name of the character, realm, and continent of the specific player's avatar. It should be colorful and have smooth finishes. | It is chosen to standardize with specific information from the video game and the avatar, that is, the statue configuration, specific name of the character or avatar, kingdom, video player's continent, all through an online digital platform. Colors are achieved simultaneously through a printer with powder sintering technology, and final finishes and touch-ups are achieved through spray painting, polishing with a rotary tool, drying in an oven, among other conventional processes. | - | ||
| GME4 | Parametric customization and individualization. | Recreation and play | ME71 | The providers offer a custom figure manufacturing service for characters from an online video game called World of Warcraft®, through the 3D printing process. | Data: A pre-existing character is chosen, customized with video game accessories to the customer's liking, and a formal request is made. Suppliers model based on the virtual model of the video game, converting it to a printable format, and manufacture it using the 3D printing process, making the respective shipment of the figure once it is manufactured. | - | - | Dimensions | It should be to scale, a desktop figure. | A scale size is chosen that is smaller than the effective volume of the powder printer. 130mm for busts and 200mm for statues. | - |
| GME4 | Parametric customization and individualization. | Recreation and play | ME71 | The providers offer a custom figure manufacturing service for characters from an online video game called World of Warcraft®, through the 3D printing process. | Data: A pre-existing character is chosen, customized with video game accessories to the customer's liking, and a formal request is made. Suppliers model based on the virtual model of the video game, converting it to a printable format, and manufacture it using the 3D printing process, making the respective shipment of the figure once it is manufactured. | - | - | Forces/Deformations | It should be rigid, it should be decorative, it will not support heavy loads. | Rigid material is chosen by powder sintering but with multiple colors, protected by a rigid plastic dome. | - |
| GME4 | Parametric customization and individualization. | Recreation and play | ME71 | The providers offer a custom figure manufacturing service for characters from an online video game called World of Warcraft®, through the 3D printing process. | Data: A pre-existing character is chosen, customized with video game accessories to the customer's liking, and a formal request is made. Suppliers model based on the virtual model of the video game, converting it to a printable format, and manufacture it using the 3D printing process, making the respective shipment of the figure once it is manufactured. | - | - | Materials | It should be rigid, it should be decorative, it will not support heavy loads. | Rigid material is chosen by powder sintering but with multiple colors, protected by a rigid plastic dome. | - |
| GME4 | Parametric customization and individualization. | Recreation and play | ME71 | The providers offer a custom figure manufacturing service for characters from an online video game called World of Warcraft®, through the 3D printing process. | Data: A pre-existing character is chosen, customized with video game accessories to the customer's liking, and a formal request is made. Suppliers model based on the virtual model of the video game, converting it to a printable format, and manufacture it using the 3D printing process, making the respective shipment of the figure once it is manufactured. | - | - | Manufacturing and Assembly | It should be rigid, it should be decorative, it will not support heavy loads. | The powder sintering process is chosen, with rigid material by powder sintering, but with multiple colors, protected by a rigid plastic dome. The final finishes are done by conventional processes. Spray painting process, polishing with rotortool, drying in oven among other conventional processes. | - |
| GME4 | Parametric customization and individualization. | Recreation and play | ME71 | The providers offer a custom figure manufacturing service for characters from an online video game called World of Warcraft®, through the 3D printing process. | Data: A pre-existing character is chosen, customized with video game accessories to the customer's liking, and a formal request is made. Suppliers model based on the virtual model of the video game, converting it to a printable format, and manufacture it using the 3D printing process, making the respective shipment of the figure once it is manufactured. | - | - | assembly | It should be composed of a platform, the figure, and the dome for protection. | The assembly of three parts is chosen, one aesthetic, and the other for support and protection. | - |
| GME4 | Parametric customization and individualization. | Recreation and play | ME71 | The providers offer a custom figure manufacturing service for characters from an online video game called World of Warcraft®, through the 3D printing process. | Data: A pre-existing character is chosen, customized with video game accessories to the customer's liking, and a formal request is made. Suppliers model based on the virtual model of the video game, converting it to a printable format, and manufacture it using the 3D printing process, making the respective shipment of the figure once it is manufactured. | - | - | Costs and Deadlines | It should be in line with a personalized product. | Statue: 130 USD + shipping, Bust: 70 USD + shipping | - |
| GME4 | Parametric customization and individualization. | Recreation and play | ME71 | The providers offer a custom figure manufacturing service for characters from an online video game called World of Warcraft®, through the 3D printing process. | Data: A pre-existing character is chosen, customized with video game accessories to the customer's liking, and a formal request is made. Suppliers model based on the virtual model of the video game, converting it to a printable format, and manufacture it using the 3D printing process, making the respective shipment of the figure once it is manufactured. | - | - | Transport and distribution | Both marketing and customization should be done remotely online. | The platform is used to create a user and carry out the entire customization and commercialization process, from the company a personalized figure is sent. | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish the WHATs of the customer, the HOWs of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and the HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Function | Avoid Migration The ideal would be for the stent to compress slightly once deployed. It should not migrate from its initial position. Migration frequently occurs as a result of insufficient stent diameter or due to tumor involution after treatment. In the trachea, in particular, displacements of up to 20% of cases can be observed if the stent has not been able to withstand. Stents with struts and partially covered metallic stents show lower migration rates than fully covered metallic stents. | A stent with a D-shaped form that resembles the natural geometry of the trachea, a reduced amount of material on the posterior wall that is in contact with the tracheal muscle wall, and a stent with rounded threads on each edge or ring. | There were 12 models after a brainstorming session in a group and the 12 designs were voted on. Manufacturing experts, designers, and doctors made the classification. Finally, only two models were selected. The design process followed an evolutionary approach with many iterations involving the opinions of customers (doctors/patients). Work was done on the final conceptual design until a satisfactory detailed design was produced. |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish THE WHYS of the customer, THE HOWS of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between THE WHYS and THE HOWS, the positive and negative correlations of THE HOWS; Third step, based on the negative correlations of THE HOWS (contradictions in the technical aspects of the solution) use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of THE HOWS; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Customization | Coincides with the patient's trachea. The stent should be adjusted to the patient for a better fit during insertion, as well as to avoid any possible migration during high expectoration. Once migration occurs, it is usually managed by extracting the migrated stent and replacing it with a new stent of a more appropriate size. Additionally, any contact pressure due to tumors can be reduced if migration is avoided by changing the position of certain stent features. The number of customization parameters should allow for proper matching between the stent and the trachea, but should not be too numerous in order to avoid complications in the automation of design. | Solidworks R linked with Excel software helped to automatically change the overall dimensions of the design for a particular patient. The selected customization parameters were those that fit the patient's trachea. The design parameters that could be modified were the stent height, internal stent diameter, external diameter, muscular wall thickness, and distance between rings. All the design rules and parameters were saved in the Excel file so that each user could modify them. Finally, with the customized stent parameters in the Excel file, a 3D stent model was created in Solidworks. | A list of attributes was made for the new stent design. They were obtained through interviews with doctors and visits to patients. Mainly, doctors provided detailed answers to the interview questions, but some patients also collaborated in this study and provided important information. Six hospitals collaborated in this study, and information was obtained from about 40 patients. Additionally, 30 doctors from these and other hospitals were interviewed to obtain a list of attributes. In general, the attributes given by the patients complement those proposed by the doctors. In this research, all attributes obtained from patients and doctors were considered equally. The attributes obtained from the interviews were supplemented with references in case not all attributes had been included. However, no other attributes were added apart from those obtained in the interviews. After the interviews, the attributes were translated back into real values that could be increased or decreased as needed. For example, 'dynamic' was expressed as reduced wall thickness. The design methodology for QFD quality was used to establish the customer's WHATs and the solution's HOWs, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one. |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish THE WHYS of the customer, THE HOWS of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between THE WHYS and THE HOWS, the positive and negative correlations of THE HOWS; Third step, based on the negative correlations of THE HOWS (contradictions in the technical aspects of the solution) use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of THE HOWS; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Customization | - | - | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish THE WHYS of the customer, THE HOWS of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between THE WHYS and THE HOWS, the positive and negative correlations of THE HOWS; Third step, based on the negative correlations of THE HOWS (contradictions in the technical aspects of the solution) use the inventive problem-solving theory TRIZ, to establish technological solutions of materials and forms, in order to solve the contradiction of THE HOWS; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design in order to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done in a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Dimensions | Dynamic. One of the main requirements of tracheal stents is the ability to easily change shape, due to expectoration but not tumor pressure. Specifically, the tracheal muscular wall undergoes high deformation during coughing and expectoration. | This deformation is attributed to its shape and thickness, which is approximately 1-1.5 mm. The natural thickness of the tracheal muscular wall should be considered a very important parameter. | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish THE WHYS of the customer, THE HOWS of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between THE WHYS and THE HOWS, the positive and negative correlations of THE HOWS; Third step, based on the negative correlations of THE HOWS (contradictions in the technical aspects of the solution) use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of THE HOWS; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Forces/Deformations | Avoid migration. | It is known that a tracheal ring has a length of 4 mm in most adults; therefore, it is an appropriate reference during validation tests to determine the permissible vertical displacement of the stent. An elastic modulus of 1-15 MPa is required to insert and remove a stent. | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish the WHATs of the customer, the HOWs of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and the HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Forces/Deformations | Dynamic. One of the main requirements of tracheal stents is the ability to easily change shape, due to expectoration but not tumor pressure. Specifically, the tracheal muscular wall undergoes high deformation during coughing and expectoration. | - | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish the WHATs of the customer, the HOWs of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and the HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Forces/Deformations | Easy to insert and remove. Easy to insert is defined as the ability of the stent to twist or adapt its shape to be inserted into a surgical instrument before an operation. | - | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish the WHATs of the customer, the HOWs of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and the HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Materials | Dynamic. One of the main requirements of tracheal stents is the ability to easily change shape, due to expectoration but not tumor pressure. Specifically, the tracheal muscular wall undergoes high deformation during coughing and expectoration. | In contrast, the wall of the tracheal stent must be rigid to prevent tumor growth and restenosis; therefore, it is strongly recommended to use a material of at least 60 Shore A. | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish the WHATs of the customer, the HOWs of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and the HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Materials | Easy to insert and remove. Easy to insert is defined as the ability of the stent to twist or adapt its shape to be inserted into a surgical instrument before an operation. | An elastic modulus of 1-15 MPa is required to insert and remove a stent. | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish the WHATs of the customer, the HOWs of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and the HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Materials | Biocompatible. Several international regulations establish the conditions and requirements of biocompatible material. | ISO 10993-1, have thermal stability at 35-45 °C. ISO 10993-18:2005: Chemical stability. | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish the WHATs of the customer, the HOWs of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and the HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Manufacturing and Assembly | It should be dimensionally accurate and handle biocompatible materials. | The final geometry of the implant was exported to the CAM program from the Fab@Home additive manufacturing technology to evaluate the geometric accuracy of the stent. | To demonstrate the reliability of the system, many tests were performed. The parameters were obtained from the computed tomography (CT) data of a 40-year-old man as shown in this work. The material used to manufacture the stents was common household silicone (Fischer Ibérica, Cambrils, Spain). |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish the WHATs of the customer, the HOWs of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and the HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done on a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | assembly | Easy to insert and remove. Easy to insert is defined as the ability of the stent to twist or adapt its shape to be inserted into a surgical instrument before an operation. Also, easy to remove is defined as the ease with which it can be removed from the body once it is no longer needed. Although both silicone and metal stents are easy to maneuver, a rigid bronchoscope often poses a serious obstacle for many endoscopists when it is necessary to quickly and effectively insert a silicone stent. The ideal stent should be removable, especially in cases of benign obstruction. In this sense, silicone stents are superior to metal stents. Metal stents cannot be easily removed because they adhere to the tracheobronchial wall within weeks. If complications arise, patients with metal stents often require multiple laser or electrocautery treatments to treat recurrent granulation caused by an irritating reaction to the metal. | An elastic modulus of 1-15 MPa is required to insert and remove a stent. | A list of attributes was made for the new stent design. They were obtained through interviews with doctors and visits to patients. Mainly, doctors provided detailed answers to the interview questions, but some patients also collaborated in this study and provided important information. Six hospitals collaborated in this study, and information was obtained from about 40 patients. Additionally, 30 doctors from these and other hospitals were interviewed to obtain a list of attributes. In general, the attributes given by the patients complement those proposed by the doctors. In this research, all attributes obtained from patients and doctors were considered equally. The attributes obtained from the interviews were supplemented with references in case not all attributes had been included. However, no other attributes were added apart from those obtained in the interviews. After the interviews, the attributes were translated back into real values that could be increased or decreased as needed. For example, 'dynamic' was expressed as reduced wall thickness. The design methodology for QFD quality was used to establish the customer's WHATs and the solution's HOWs, their respective evaluation, benchmarking of the competition, the level of correlation between the WHATs and HOWs, the positive and negative correlations of the HOWs; Third step, based on the negative correlations of the HOWs (contradictions in the technical aspects of the solution), use the TRIZ inventive problem-solving theory to establish technological solutions for materials and forms, in order to resolve the contradictions of the HOWs; Fourth step, verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one. |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish THE WHYS of the customer, THE HOWS of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between THE WHYS and THE HOWS, the positive and negative correlations of THE HOWS; Third step, based on the negative correlations of THE HOWS (contradictions in the technical aspects of the solution) use the inventive problem-solving theory TRIZ, to establish technological solutions of materials and forms, in order to solve the contradiction of THE HOWS; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design in order to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done in a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | assembly | - | - | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish THE WHYS of the customer, THE HOWS of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between THE WHYS and THE HOWS, the positive and negative correlations of THE HOWS; Third step, based on the negative correlations of THE HOWS (contradictions in the technical aspects of the solution) use the inventive problem-solving theory TRIZ, to establish technological solutions of materials and forms, in order to solve the contradiction of THE HOWS; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design in order to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done in a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Legal Aspects | Biocompatible. Several international regulations establish the conditions and requirements of biocompatible material. A material will be considered biocompatible if it allows the body to function without any complications such as chronic inflammation prolonged at the point of contact, the generation of toxic materials for cells, also known as cytotoxicity, cellular alteration, skin irritation, restenosis, implant corrosion, and the formation of granulation tissue. | ISO 10993-1, The FDA approved long-term implantation, The material must have thermal stability at 35-45 °C. ISO 10993-18:2005: Chemical stability, should not react with the environment (air, body cells, blood) | - |
| GME4 | Parametric customization and individualization. | trachea implant | 2 | present an integrated tool for designing a tracheal stent that meets the most important requirements or needs of doctors, hospital administrators, and patients | First step, Interviews with patients and medical staff in order to create a list of product attributes; Second step, based on the list of product attributes, the QFD design methodology was used to establish THE WHYS of the customer, THE HOWS of the solutions, their respective evaluation, benchmarking of the competition, the level of correlation between THE WHYS and THE HOWS, the positive and negative correlations of THE HOWS; Third step, based on the negative correlations of THE HOWS (contradictions in the technical aspects of the solution) use the inventive problem-solving theory TRIZ, to establish technological solutions of materials and forms, in order to solve the contradiction of THE HOWS; Fourth step, Verify if all customer requirements are met; Fifth step, evaluate the proposed solutions and select the best one; Sixth step, parameterize the solution; Seventh step, implement the concept through additive manufacturing AM. For the last two steps, the Solidwork® and Excel® software were used to parameterize the design in order to consider the specific measurements of the patient extracted from the computed tomography of the same, the manufacturing was done in a Fab@home machine, with homemade silicone. The methodology can be implemented considering other more precise AM processes and bio-compatible materials. | The result is a stent form approved by doctors. In addition, a parameterization tool was used to modify the overall dimensions of the stent to fit a specific patient. Finally, the final conceptual design was printed using an additive manufacturing technology known as Fab@Home. | Combine and customize various well-known design techniques for their application in the development of tracheal endoprosthesis devices, a new approach to device development in the field of medicine. Other tracheal endoprosthesis devices are customized but not personalized because the design techniques used do not allow detailing the geometric characteristics at this level as the presented approach does. It meets two main requirements: a wavy D shape intended to prevent migration and a thickness of the back wall that can improve tracheal dynamics and prevent obstruction. However, future work is needed to validate the manufacturing technique, material biocompatibility, and the efficiency of the entire process. Consider packaging, sterilization, and labeling at an early stage as they are integral parts of the entire device. | Legal Aspects | ISO 10993-1, have thermal stability at 35-45 °C. ISO 10993-18:2005: Chemical stability. | - | - |
| GME4 | Parametric customization and individualization. | furniture, prosthesis | 45/ 163 /164 | Develop a set of new formal design representations that capture design requirements to enhance the level of customization while leveraging the design freedom enabled by additive manufacturing. | Formal design process structure that merges design for customization and design for additive manufacturing. Formal representations for artifact-user interaction using finite state automata and user goal-oriented behaviors in artifact use as sets of formal language based on discrete event systems. By adopting the relational properties of affordability, effectiveness, and preference, the proposed formal representations systematically link artifact properties related to user behavior and preferences with the design requirements of additive manufacturing artifacts [45]. | The main contribution of this research is the development of a formal design process and the design of knowledge representations for AM-enabled personalization. So far, the proposed method focuses mainly on conceptual and preliminary design. A case study based on the design of a chair [45] is analyzed. Among the results, it deals with the availability of the methodology in the design of additionally manufactured interactive products, such as prosthetics [163]. | A formal framework for a design process for AM-facilitated customization and relevant design knowledge representations was proposed. The process structure showed a way to systematically integrate the concepts of affordability, effectiveness, preference, and user behavior into the frameworks of DFP and DFAM to leverage the design freedom enhanced by AM. The AEP-based FSA was proposed as a formal representation of artifact-user interaction. Formal representations of user behaviors in artifact use were then introduced as sets of user actions and sets of formal language [45]. | - | - | - | - |
| GME4 | Parametric customization and individualization. | 53 | This article presents a variety of product design processes for different levels of customization through the use of AM, from processes of elaborate individualization to specific product adaptation. | Basically, the article presents a methodology with 4 criteria for deciding whether to redesign parts of product series using AM (replacing parts produced with traditional manufacturing). 4 cases are presented. The criteria are: Integrated design, which means that there are multiple functions in one or few parts, allowing for a more compact product and a reduced number of parts; Individualization, or customization of parts that allows for meeting customer needs, which implies a greater variation in sizes, but to compensate for this, the parts are divided into standardized and customized ones, with the standardized ones still being manufactured using traditional methods, but the customized ones being manufactured using AM; Lightweight design, if the product is in motion or has dynamic applications, reducing the weight will improve performance; Efficient design, which means improving the product's efficiency in operation. | Examples of redesigned parts for AM are presented: medical device (reduction of parts), individualized pipe inspection element, lighter aerial support, among others. | Among the conclusions, it is highlighted that the redesign should not be focused on a single objective. | - | - | - | - | |
| GME4 | Parametric customization and individualization. | 83 | To contribute to the design of individualized products and answer the following research question: What processes can lead to design through the use of AM for customization? | A systematic literature review is carried out to examine existing approaches to implementing AM. The literature on design processes in custom design, the effects of production on the design phase, the use of AM in production, and the application of AM for customization was examined. After collecting the data, the types of design processes for customization through AM are concluded. The synthesis of the processes results, in particular, in adaptations of the processes of established design approaches. | Standardized individualization is recommended for personalization with AM for components where geometric individualization can increase customer satisfaction, comfort, or product applicability. It is recommended when the shape of the product can be adapted to individuals. It is useful if the contact surfaces are known and specific customer data can be measured. The process for a typical individualization case should be organized, standardized, and capable of automation. The source and type of data, limits of individual geometry, fabricability, and interfaces with other components are taken into account during the development of the recurring personalization process. Specific adaptation, the product can be adapted to customer desires within a fixed solution space or individual adaptations for design or engineering without changing the main attributes of the product or requiring a completely new engineering product. A product structure design for product adaptation is executed: an individualization scope is defined, enclosing the central product structure with customizable features and an open zone for individualization. The disadvantage of specific adaptation is the significant effort required in the structural and conceptual design of the preceding product, in which an independent spectrum of user-independent products and the basic potential of the product are developed. | Possible different customization strategies are possible. With attention focused on the influence in the design process, this document identifies a series of customization characteristics. Two types of customization were presented in the made-to-order manufacturing environment: standardized individualization, with a previous process development, resulting in a defined degree of customization for user participation; specific sample adaptation that the client desires influences the previous steps of the design process. Different levels of customer participation require different design methods and adaptation of individualization processes for differentiation in this field to make sense. It is emphasized that automated processes are required in the design of individualized products, with an emphasis on standards and programs, specific design methods are also required for customized products. | - | - | - | - | |
| GME4 | Parametric customization and individualization. | Recreation and Play, tools, spare parts | 280 | Shed light on the remixing mechanisms. To what extent can measurable evidence of the expected positive outcomes of remixing be found? | In a first step, we investigate qualitative data from 81 individual designs based on remixing to identify the underlying mechanisms of remixing. We identified six of these mechanisms that can also be grouped by the tendential outcome of the respective process (creativity-oriented: inspiration, play, learning; productivity-oriented: speed, improvement, enhancement). In a second stage, we move on to a quantitative analysis of the platform data, which indicates that remixing can lead to better design process outcomes in terms of quantity and diversity of designs. | The designers who take advantage of the remixing features are active in more categories and show less specialization than their non-remixing peers. These results demonstrate that the remixing mechanisms for inspiration and remixing for empowerment are manifested very appropriately in the platform-level results. Remixing intervention allows designers to cover more ground more quickly. Remixing designers also create more designs per designer, providing strong evidence for the effectiveness of the remixing mechanism for speed. With regard to quality, a larger proportion of designs created by remixers are printed by community members. This higher printing rate tends to improve, so designers optimize products with respect to functional attributes and in turn make them more 'printable'. Remixing for empowerment is the third channel focused on productivity and describes how designers rely on existing solutions to achieve the desired outcome. Here, designers use intervention as a temporary solution without which they could not achieve the desired solution. | Almost all innovations arise as recombination of existing building blocks. Consequently, remixing can be found everywhere, at least at an abstract level. Therefore, any structure that supports this natural process will inevitably enhance inventiveness. | Function / Customization | You must combine different features or solutions from different designs to a personalized problem | The database to inspire the integrated solution is a digital thinkgiver database, which contains 1.6 million unique designs with popularity statistics, number of impressions, and downloads. It can inspire from various models or design a single design by combining multiple designs (remix). | The designers who take advantage of the remixing features are active in more categories. The remixing intervention allows designers to cover more ground more quickly. Remixing designers also create more designs per designer, providing strong evidence for the effectiveness of the remixing mechanism for speed. |
| GME4 | Parametric customization and individualization. | Recreation and Play, tools, spare parts | 280 | Shed light on the remixing mechanisms. To what extent can measurable evidence of the expected positive outcomes of remixing be found? | In a first step, we investigate qualitative data from 81 individual designs based on remixing to identify the underlying mechanisms of remixing. We identified six of these mechanisms that can also be grouped by the tendential outcome of the respective process (creativity-oriented: inspiration, play, learning; productivity-oriented: speed, improvement, enhancement). In a second stage, we move on to a quantitative analysis of the platform data, which indicates that remixing can lead to better design process outcomes in terms of quantity and diversity of designs. | The designers who take advantage of the remixing features are active in more categories and show less specialization than their non-remixing peers. These results demonstrate that the remixing mechanisms for inspiration and remixing for empowerment are manifested very appropriately in the platform-level results. Remixing intervention allows designers to cover more ground more quickly. Remixing designers also create more designs per designer, providing strong evidence for the effectiveness of the remixing mechanism for speed. With regard to quality, a larger proportion of designs created by remixers are printed by community members. This higher printing rate tends to improve, so designers optimize products with respect to functional attributes and in turn make them more 'printable'. Remixing for empowerment is the third channel focused on productivity and describes how designers rely on existing solutions to achieve the desired outcome. Here, designers use intervention as a temporary solution without which they could not achieve the desired solution. | Almost all innovations arise as recombination of existing building blocks. Consequently, remixing can be found everywhere, at least at an abstract level. Therefore, any structure that supports this natural process will inevitably enhance inventiveness. | Function / Customization | You must allow the selection of final layout configuration. I have removed the quotation and double quotation marks from the translated value. | Each design of the base can be constituted as a configuration that is selected within the design process. | Almost all innovations arise as recombination of existing building blocks. |
| GME4 | Parametric customization and individualization. | Recreation and Play, tools, spare parts | 280 | Shed light on the remixing mechanisms. To what extent can measurable evidence of the expected positive outcomes of remixing be found? | In a first step, we investigate qualitative data from 81 individual designs based on remixing to identify the underlying mechanisms of remixing. We identified six of these mechanisms that can also be grouped by the tendential outcome of the respective process (creativity-oriented: inspiration, play, learning; productivity-oriented: speed, improvement, enhancement). In a second stage, we move on to a quantitative analysis of the platform data, which indicates that remixing can lead to better design process outcomes in terms of quantity and diversity of designs. | The designers who take advantage of the remixing features are active in more categories and show less specialization than their non-remixing peers. These results demonstrate that the remixing mechanisms for inspiration and remixing for empowerment are manifested very appropriately in the platform-level results. Remixing intervention allows designers to cover more ground more quickly. Remixing designers also create more designs per designer, providing strong evidence for the effectiveness of the remixing mechanism for speed. With regard to quality, a larger proportion of designs created by remixers are printed by community members. This higher printing rate tends to improve, so designers optimize products with respect to functional attributes and in turn make them more 'printable'. Remixing for empowerment is the third channel focused on productivity and describes how designers rely on existing solutions to achieve the desired outcome. Here, designers use intervention as a temporary solution without which they could not achieve the desired solution. | Almost all innovations arise as recombination of existing building blocks. Consequently, remixing can be found everywhere, at least at an abstract level. Therefore, any structure that supports this natural process will inevitably enhance inventiveness. | Manufacturing and Assembly | Apart from ensuring the feasibility of the function, it must also ensure the manufacturability of the solution. | The think giverse platform contains statistics on the number of file downloads, and these contain observations about manufacturing and the number of times they have been printed. | Regarding quality, a larger part of the designs created by remixers are printed by community members. Designers optimize products in terms of functional attributes and make them more printable. This higher printing rate tends to improve remixing for empowerment as the third channel focused on productivity and describes how designers rely on existing solutions to achieve the desired outcome. |
| GME4 | Parametric customization and individualization. | tissue implant | ME24 | We demonstrate how to manufacture the mold of soft prostheses with a low-cost desktop 3D printer. | The manufacturing method used is known as Scanning Printing Polishing Casting (SPPC). First, the anatomy is scanned with a 3D scanner (kinet for Windows), then a tissue casting mold is designed on the computer (Rhinoceros V4.0 software) and printed in ABS with a desktop 3D printer (FFF). Subsequently, a chemical polishing method is used (with acetone, the heater is turned on, the container containing the acetone is heated to a temperature higher than the boiling point of acetone (56.5°C), as it is set to 80°C for the experiment. The heated acetone vapor rises from the container and washes the ABS sample, the acetone will no longer evaporate when the pressure inside the sealed container reaches its saturated vapor pressure. A simple equation (Ideal Gas State Equation) was used to calculate the reasonable volume of acetone) to polish the casting mold, removing the stair-step effect and acquiring a smooth surface. Finally, the last step is to melt medical-grade silicone into the mold (Silicone part A and part B (Dongguan Hongfeng Silicone Materials Co., LTD) were mixed in a 1:1 ratio (weight:weight) and stirred for 2 minutes, and then the mixture was degassed in a vacuum chamber for 10-15 minutes). After the silicone is cured, the soft prostheses can be removed from the mold. Using the SPPC method, soft prostheses with a smooth surface and complicated structure can be manufactured at a low cost. | Manufacture soft prostheses with the help of a desktop 3D printer. The printing costs of the negative mold were insignificant compared to traditional methods. In this report, a cheap and convenient method (SPPC) for manufacturing silicone prostheses is demonstrated. The staircase effect caused by the desktop 3D printer is eliminated. the translation may vary depending on the context and specific language nuances. | As an AI language model, I can help you with translation. Here is the translation of the provided data into English: As a result, the total cost of manufacturing the ear prosthesis is around S30, which is much lower than current methods of manufacturing soft prostheses. the translation may not be 100% accurate, as it is generated by an AI model. | - | - | - | - |
| GME4 | Parametric customization and individualization. | surgical instrument, instrument prototype | ME74 | This concept of custom surgical instruments was investigated as a pilot demonstration of mass customization and on-demand manufacturing by a team from the Naval Postgraduate Dental School (Bethesda, MD), Stratasys (Eden Prairie, MN), and the Defense Advanced Research Projects Agency (Arlington, VA) Service Chiefs Fellowship Program. Spring devices for custom orthodontic brackets and customized pivot pliers were digitally designed, manufactured, and evaluated using a surgical simulator. | The digital models of a spring clamp and a central pivot clamp were created in the SolidWorks 2012 computer-aided design (CAD) software. The models were parameterized with key dimensions (arm lengths, finger loop positions, etc.). Custom designs were generated simply by changing the values of the key dimensions within the CAD application. The instruments were sized to fit the hands of a clinician and tailored to express personal preferences and preferred technique. It was necessary to adapt the designs to accommodate the characteristics of currently available materials; this involved thickening the cross-sectional area of the clamp arms and redesigning the simple pivot hinges to provide the appropriate mechanical strength and rigidity. A Stratasys ABSi-Ag development material was used to print examples of the instruments. This material is currently being developed as a potentially biocompatible and bacteriostatic material for medical applications. Custom tissue forceps and a needle driver were designed, manufactured, and tested according to the physician's personal specifications. A subjective evaluation was performed by performing surgical procedures and suturing an incision on a surgical simulator of a cut suit. | A one-piece, spring-style tissue forceps design was created in CAD. The design was custom-made to fit the hand of a clinician, and later adapted in stiffness to provide the desired sensation with palm pressure. Interlocking triangular teeth were added to the tip of the instrument, according to the clinician's preference. Simple grip and hold tests were performed to evaluate the design; the information provided by the clinician led to further modifications. The stiffness of the arms, the length of the arms, and the angle of the arms in closure were modified at the request of the clinician. The change in the material deposition pattern in the material extrusion process affected the instrument's sensation as reported by the clinician. A two-piece hinge pivot forceps design was also created in CAD. A standard pivoting arm design, common to hemostats, was modified for production in ABS plastic. A 'T'-shaped pivot key and a tapered slot joined the arms. Handling dimensions included arm length, arm section, jaw length, jaw section, finger loop diameter, and position. The clinician expressed a desire for an instrument that could be picked up, manipulated, and dropped from a gloved hand with minimal manual articulation. The serrated jaws were sized for dissection and holding tasks. A closing zipper tooth was added to the back of the arm handles for closure; the zipper was operated by palm pressure. A basic surgery kit was printed on the FDM device, including tissue forceps, hemostat, and needle holders; the printing time was just over 6 hours. Successful laparotomy, ligation, splenectomy, and suturing were performed in the cutting suit using the custom instruments. | The instruments of spring and pivot pliers were designed according to the specifications of a clinical test, adapted to the dimensions of the hand, personal preference for the instrument's feel, and preferred surgical technique. The instruments were 3D printed on demand using a material extrusion 3D printer and then successfully used to complete surgical procedures on a realistic human simulator. With the development of new biocompatible materials for additive manufacturing, it will be possible to 3D print custom surgical instruments on demand. With design and manufacturing cycles of just a few hours, it will be feasible to quickly develop new instruments tailored to new surgical techniques and procedures. | - | - | - | - |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME35 | The purpose of this pilot study was to determine if the printed surgical instruments would withstand chemical sterilization and the stress of an operation. | Using a fused deposition modeling printer, a surgical retractor from the Army/Navy was replicated using polylactic acid (PLA) filament. The retractor was sterilized using standard glutaraldehyde protocols approved by the Food and Drug Administration, bacteria presence was tested using polymerase chain reaction, and it was subjected to tension until fracture to determine if the printed instrument could withstand a force greater than that required in an operating room. | The print took approximately 90 minutes. The instrument tolerated 13.6 kg of tangential force before failing, both before and after exposure to the sterilant. The freshly extruded PLA from the printer was sterile and did not produce any polymerase chain reaction products. Each instrument weighed 16 g and required only $0.46 worth of PLA. | Our estimates place the cost per unit of a 3D printed retractor at approximately one-tenth of the cost of a stainless steel instrument. The Army and Navy retractor is strong enough for the demands of the operating room. The freshly extruded PLA in a clean environment, such as an operating room, would produce a sterile instrument ready for use. Due to the unprecedented accessibility of 3D printing technology worldwide and the cost efficiency of these instruments, there are far-reaching implications for surgery in some underserved and less developed parts of the world. | Function | It must support operating room loads, It must be hypoallergenic and must withstand chemical sterilization processes. | An instrument similar to stainless steel ones is chosen, modified to withstand loads with PLA material and after manufacturing undergoes sterilization with glutaraldehyde at room temperature. | Data: Obedece a búsqueda bibliográfica. Derivada en la esterilización por glutaraldehido. [6] Fedorovich NE, Alblas J, Hennink WE, et al. Organ printing: the future of bone regeneration? Trends Biotechnol 2011;29:601. Y respecto a la carga y Resistencia [7] Kondor S, Grant G, Liacouras P, et al. On demand additive manufacturing of a basic surgical kit. J Med Devices J Med Devices 2013;7:030916. It follows a bibliographic search. Derived in sterilization by glutaraldehyde. [6] Fedorovich NE, Alblas J, Hennink WE, et al. Organ printing: the future of bone regeneration? Trends Biotechnol 2011;29:601. And regarding the load and resistance [7] Kondor S, Grant G, Liacouras P, et al. On-demand additive manufacturing of a basic surgical kit. J Med Devices J Med Devices 2013;7:030916. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME35 | The purpose of this pilot study was to determine if the printed surgical instruments would withstand chemical sterilization and the stress of an operation. | Using a fused deposition modeling printer, a surgical retractor from the Army/Navy was replicated using polylactic acid (PLA) filament. The retractor was sterilized using standard glutaraldehyde protocols approved by the Food and Drug Administration, bacteria presence was tested using polymerase chain reaction, and it was subjected to tension until fracture to determine if the printed instrument could withstand a force greater than that required in an operating room. | The print took approximately 90 minutes. The instrument tolerated 13.6 kg of tangential force before failing, both before and after exposure to the sterilant. The freshly extruded PLA from the printer was sterile and did not produce any polymerase chain reaction products. Each instrument weighed 16 g and required only $0.46 worth of PLA. | Our estimates place the cost per unit of a 3D printed retractor at approximately one-tenth of the cost of a stainless steel instrument. The Army and Navy retractor is strong enough for the demands of the operating room. The freshly extruded PLA in a clean environment, such as an operating room, would produce a sterile instrument ready for use. Due to the unprecedented accessibility of 3D printing technology worldwide and the cost efficiency of these instruments, there are far-reaching implications for surgery in some underserved and less developed parts of the world. | Dimensions | It must have dimensions similar to stainless steel retractors. | The translated value of El instrumento medía 17 cm 1,5 cm 4 mm in English is The instrument measured 17 cm 1.5 cm 4 mm. | Similar dimensions to the original retractor. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME35 | The purpose of this pilot study was to determine if the printed surgical instruments would withstand chemical sterilization and the stress of an operation. | Using a fused deposition modeling printer, a surgical retractor from the Army/Navy was replicated using polylactic acid (PLA) filament. The retractor was sterilized using standard glutaraldehyde protocols approved by the Food and Drug Administration, bacteria presence was tested using polymerase chain reaction, and it was subjected to tension until fracture to determine if the printed instrument could withstand a force greater than that required in an operating room. | The print took approximately 90 minutes. The instrument tolerated 13.6 kg of tangential force before failing, both before and after exposure to the sterilant. The freshly extruded PLA from the printer was sterile and did not produce any polymerase chain reaction products. Each instrument weighed 16 g and required only $0.46 worth of PLA. | Our estimates place the cost per unit of a 3D printed retractor at approximately one-tenth of the cost of a stainless steel instrument. The Army and Navy retractor is strong enough for the demands of the operating room. The freshly extruded PLA in a clean environment, such as an operating room, would produce a sterile instrument ready for use. Due to the unprecedented accessibility of 3D printing technology worldwide and the cost efficiency of these instruments, there are far-reaching implications for surgery in some underserved and less developed parts of the world. | Strength | Support the loads during operation. | To simulate load, a 5cm belt is used, with a tangential load of 11.3+0.57 kg until deformation, 13.6+0.68kg until rupture at 15.9+0.8kg, both for sterilized and non-sterilized instruments. | An experiment is carried out to characterize the resistance of the instrument, both for the sterilized and non-sterilized instrument. The load is fixed with the help of bibliographic reference No. [Ref.7] of an article that specifies the load on retraction of flaps in thoracic and abdominal operations. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME35 | The purpose of this pilot study was to determine if the printed surgical instruments would withstand chemical sterilization and the stress of an operation. | Using a fused deposition modeling printer, a surgical retractor from the Army/Navy was replicated using polylactic acid (PLA) filament. The retractor was sterilized using standard glutaraldehyde protocols approved by the Food and Drug Administration, bacteria presence was tested using polymerase chain reaction, and it was subjected to tension until fracture to determine if the printed instrument could withstand a force greater than that required in an operating room. | The print took approximately 90 minutes. The instrument tolerated 13.6 kg of tangential force before failing, both before and after exposure to the sterilant. The freshly extruded PLA from the printer was sterile and did not produce any polymerase chain reaction products. Each instrument weighed 16 g and required only $0.46 worth of PLA. | Our estimates place the cost per unit of a 3D printed retractor at approximately one-tenth of the cost of a stainless steel instrument. The Army and Navy retractor is strong enough for the demands of the operating room. The freshly extruded PLA in a clean environment, such as an operating room, would produce a sterile instrument ready for use. Due to the unprecedented accessibility of 3D printing technology worldwide and the cost efficiency of these instruments, there are far-reaching implications for surgery in some underserved and less developed parts of the world. | Material | It should be made of hypoallergenic and sterilized material, and with sufficient resistance. | PLA material and glutaraldehyde sterilization at room temperature | Based on bibliographic reference, sterilization by autoclave is ruled out due to degradation of resistance at high temperature, and sterilization with ethylene oxide gas is also ruled out, as the harmful levels of ethylene oxide residues are a serious concern, although it does not affect the resistance of PLA. Previously, the effectiveness of sterilization of PLA with glutaraldehyde had been tested [ref. 11]. The sole extrusion temperature during manufacturing, which is 240°C, is sufficient to sterilize the instruments if combined with sterile conditions during manufacturing. Chemical sterilization left bacterial bodies on the instruments that were still detectable by the test, further research is required in that regard. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME35 | The purpose of this pilot study was to determine if the printed surgical instruments would withstand chemical sterilization and the stress of an operation. | Using a fused deposition modeling printer, a surgical retractor from the Army/Navy was replicated using polylactic acid (PLA) filament. The retractor was sterilized using standard glutaraldehyde protocols approved by the Food and Drug Administration, bacteria presence was tested using polymerase chain reaction, and it was subjected to tension until fracture to determine if the printed instrument could withstand a force greater than that required in an operating room. | The print took approximately 90 minutes. The instrument tolerated 13.6 kg of tangential force before failing, both before and after exposure to the sterilant. The freshly extruded PLA from the printer was sterile and did not produce any polymerase chain reaction products. Each instrument weighed 16 g and required only $0.46 worth of PLA. | Our estimates place the cost per unit of a 3D printed retractor at approximately one-tenth of the cost of a stainless steel instrument. The Army and Navy retractor is strong enough for the demands of the operating room. The freshly extruded PLA in a clean environment, such as an operating room, would produce a sterile instrument ready for use. Due to the unprecedented accessibility of 3D printing technology worldwide and the cost efficiency of these instruments, there are far-reaching implications for surgery in some underserved and less developed parts of the world. | Manufacturing and Assembly | It should be an economic and fast process compared to the original process, as well as accessible. | FDM/Stratasys is selected followed by a chemical sterilization process with glutaraldehyde. | The translated value of El proceso FDM es accesible y relativamente económico in English is The FDM process is accessible and relatively economical. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME35 | The purpose of this pilot study was to determine if the printed surgical instruments would withstand chemical sterilization and the stress of an operation. | Using a fused deposition modeling printer, a surgical retractor from the Army/Navy was replicated using polylactic acid (PLA) filament. The retractor was sterilized using standard glutaraldehyde protocols approved by the Food and Drug Administration, bacteria presence was tested using polymerase chain reaction, and it was subjected to tension until fracture to determine if the printed instrument could withstand a force greater than that required in an operating room. | The print took approximately 90 minutes. The instrument tolerated 13.6 kg of tangential force before failing, both before and after exposure to the sterilant. The freshly extruded PLA from the printer was sterile and did not produce any polymerase chain reaction products. Each instrument weighed 16 g and required only $0.46 worth of PLA. | Our estimates place the cost per unit of a 3D printed retractor at approximately one-tenth of the cost of a stainless steel instrument. The Army and Navy retractor is strong enough for the demands of the operating room. The freshly extruded PLA in a clean environment, such as an operating room, would produce a sterile instrument ready for use. Due to the unprecedented accessibility of 3D printing technology worldwide and the cost efficiency of these instruments, there are far-reaching implications for surgery in some underserved and less developed parts of the world. | Costs and deadlines | It should be an economic and fast process compared to the original process. | The cost is 1/10 of the stainless steel instrument, and a manufacturing time of less than 90 minutes. | An economic evaluation is carried out for the two processes: For example, a new set of two stainless steel retractors from the Army/Navy is available online for a retail price of $46.96, making the unit cost $23.48. Our 3D printer is available for $2199 and 1 kg of PLA is available for $27.99 including shipping. Since each retractor weighs 16 g, we can make 61 retractors/kg, which calculates to $0.46 of PLA per instrument. We would need to print 95 retractors to cover the cost of the printer and make each unit cost the same as the stainless steel version, $23.48. If our printer operated at 95% efficiency during the following week (168 h), the printer would pay for itself. Even if these instruments were used only once, they would still be less expensive than the cost of damage or theft of the steel instruments. In addition, 3D printers are quite durable. Our printer has completed a moderate 2000 h of printing without significant hardware failures and standard regular maintenance. If we were to spend the last 2.7 months, approximately 2000 h, printing retractors at 95% efficiency, we would have 950 retractors with a unit cost of $2.77. Not only does the low cost of this new technology make it accessible, but the potential cost savings can make it fiscally responsible. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME35 | The purpose of this pilot study was to determine if the printed surgical instruments would withstand chemical sterilization and the stress of an operation. | Using a fused deposition modeling printer, a surgical retractor from the Army/Navy was replicated using polylactic acid (PLA) filament. The retractor was sterilized using standard glutaraldehyde protocols approved by the Food and Drug Administration, bacteria presence was tested using polymerase chain reaction, and it was subjected to tension until fracture to determine if the printed instrument could withstand a force greater than that required in an operating room. | The print took approximately 90 minutes. The instrument tolerated 13.6 kg of tangential force before failing, both before and after exposure to the sterilant. The freshly extruded PLA from the printer was sterile and did not produce any polymerase chain reaction products. Each instrument weighed 16 g and required only $0.46 worth of PLA. | Our estimates place the cost per unit of a 3D printed retractor at approximately one-tenth of the cost of a stainless steel instrument. The Army and Navy retractor is strong enough for the demands of the operating room. The freshly extruded PLA in a clean environment, such as an operating room, would produce a sterile instrument ready for use. Due to the unprecedented accessibility of 3D printing technology worldwide and the cost efficiency of these instruments, there are far-reaching implications for surgery in some underserved and less developed parts of the world. | Security | It should be made of hypoallergenic material and sterilized according to sanitary product standards. | Subject to a chemical sterilization process with glutaraldehyde after printing | The sterilization involved immersion in a 2.4% glutaraldehyde solution with a pH of 7.5 for 20 minutes at 25°C, according to the CDC guidelines for critical medical devices [6]. All samples were subjected to binary load testing using polymerase chain reaction (PCR) amplification of the V1-V2 region of the 16s rRNA gene as a measure of intact bacterial DNA. Briefly, 200 mL of sterile phosphate-buffered saline solution was added to each sample and vortexed. Two microliters of buffer were used as a template in a PCR consisting of 4 minutes at 98°C followed by 30 cycles of 98°C for 10 s, 68.8°C for 30 s, 72°C for 30 s. The PCR reagents were obtained from the Applied Biosystems real-time PCR master mix (Grand Island, NY) with 2 U of Phusion polymerase (New England Biolabs Incorporated, Ipswich, MA). The forward primer sequence was: AGAGTTT GATCMTGGCTCAG and the reverse primer sequence was: CYIACTGCTGCCTCCCGTAG. Two microliters of the resulting PCR product from each reaction were analyzed on an agarose gel to determine if an anticipated PCR product had formed. Negative controls consisting of purified water were included to monitor reagent contamination. A positive control containing Escherichia coli genomic DNA was included to demonstrate the success of the procedure. The sole extrusion temperature during manufacturing, which is 240°C, is sufficient to sterilize the instruments. If combined with sterile conditions during manufacturing, chemical sterilization left bacterial residues on the instruments still detectable by the test. Further research is required in this regard. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME35 | The purpose of this pilot study was to determine if the printed surgical instruments would withstand chemical sterilization and the stress of an operation. | Using a fused deposition modeling printer, a surgical retractor from the Army/Navy was replicated using polylactic acid (PLA) filament. The retractor was sterilized using standard glutaraldehyde protocols approved by the Food and Drug Administration, bacteria presence was tested using polymerase chain reaction, and it was subjected to tension until fracture to determine if the printed instrument could withstand a force greater than that required in an operating room. | The print took approximately 90 minutes. The instrument tolerated 13.6 kg of tangential force before failing, both before and after exposure to the sterilant. The freshly extruded PLA from the printer was sterile and did not produce any polymerase chain reaction products. Each instrument weighed 16 g and required only $0.46 worth of PLA. | Our estimates place the cost per unit of a 3D printed retractor at approximately one-tenth of the cost of a stainless steel instrument. The Army and Navy retractor is strong enough for the demands of the operating room. The freshly extruded PLA in a clean environment, such as an operating room, would produce a sterile instrument ready for use. Due to the unprecedented accessibility of 3D printing technology worldwide and the cost efficiency of these instruments, there are far-reaching implications for surgery in some underserved and less developed parts of the world. | Legal Aspects | It must be sterilized according to CDC guidelines for critical medical devices | Specific bibliographic reference guidelines are followed (Ref 6 from the bibliography, Fedorovich NE, Alblas J, Hennink WE, et al. Organ printing: the future of bone regeneration? Trends Biotechnol 2011;29:601) | Obey the bibliographic search. Derived in sterilization by glutaraldehyde. [6] Fedorovich NE, Alblas J, Hennink WE, et al. Organ printing: the future of bone regeneration? Trends Biotechnol 2011;29:601. |
| GME5 | Surgical tools and prototypes | Surgical instrument, instrument prototype, biomodels, prostheses, implants | ME38 | The objective of this article was to review the current applications of 3DP in modern surgical practice. | A electronic search was conducted in MEDLINE, EMBASE and PsycINFO for terms related to 3DP. These were then examined for their relevance and the practical applications of the technology in surgery. | Results: Initially, 488 articles were found, and these were finally reduced to 93 full-text articles. It was determined that there were three main areas in which technology is being used for printing: (1) anatomical models, (2) surgical instruments, and (3) implants and prostheses. | The different specialties are at different stages in the use of technology. The costs of implementing technology and the time it takes for printing are important factors to consider before widespread use. In the foreseeable future, it is an exciting and interesting technology with the ability to radically change healthcare and revolutionize modern surgery. | Function | You must guide and position the drilling properly. | Custom-made templates | Using reconstructed images by CT scan to create a guide that contours around the bone, allowing drilling up to a predetermined point (labeled in preoperative 3D images) during the placement of pedicle screws. |
| GME5 | Surgical tools and prototypes | Surgical instrument, instrument prototype, biomodels, prostheses, implants | ME38 | The objective of this article was to review the current applications of 3DP in modern surgical practice. | A electronic search was conducted in MEDLINE, EMBASE and PsycINFO for terms related to 3DP. These were then examined for their relevance and the practical applications of the technology in surgery. | Results: Initially, 488 articles were found, and these were finally reduced to 93 full-text articles. It was determined that there were three main areas in which technology is being used for printing: (1) anatomical models, (2) surgical instruments, and (3) implants and prostheses. | The different specialties are at different stages in the use of technology. The costs of implementing technology and the time it takes for printing are important factors to consider before widespread use. In the foreseeable future, it is an exciting and interesting technology with the ability to radically change healthcare and revolutionize modern surgery. | Customization | It must be adapted to the patient's geometry in size and shape. | Using 3D reconstructed images by CT scan. | Tagged in 3D images taken preoperatively. They require more image and data processing to produce the printed object. |
| GME5 | Surgical tools and prototypes | Surgical instrument, instrument prototype, biomodels, prostheses, implants | ME38 | The objective of this article was to review the current applications of 3DP in modern surgical practice. | A electronic search was conducted in MEDLINE, EMBASE and PsycINFO for terms related to 3DP. These were then examined for their relevance and the practical applications of the technology in surgery. | Results: Initially, 488 articles were found, and these were finally reduced to 93 full-text articles. It was determined that there were three main areas in which technology is being used for printing: (1) anatomical models, (2) surgical instruments, and (3) implants and prostheses. | The different specialties are at different stages in the use of technology. The costs of implementing technology and the time it takes for printing are important factors to consider before widespread use. In the foreseeable future, it is an exciting and interesting technology with the ability to radically change healthcare and revolutionize modern surgery. | Dimensions | It must be adapted to the patient's geometry in size and shape. | Reconstructed 3D CT images | Tagged in 3D images taken preoperatively. They require more image and data processing to produce the printed object. |
| GME5 | Surgical tools and prototypes | Surgical instrument, instrument prototype, biomodels, prostheses, implants | ME38 | The objective of this article was to review the current applications of 3DP in modern surgical practice. | A electronic search was conducted in MEDLINE, EMBASE and PsycINFO for terms related to 3DP. These were then examined for their relevance and the practical applications of the technology in surgery. | Results: Initially, 488 articles were found, and these were finally reduced to 93 full-text articles. It was determined that there were three main areas in which technology is being used for printing: (1) anatomical models, (2) surgical instruments, and (3) implants and prostheses. | The different specialties are at different stages in the use of technology. The costs of implementing technology and the time it takes for printing are important factors to consider before widespread use. In the foreseeable future, it is an exciting and interesting technology with the ability to radically change healthcare and revolutionize modern surgery. | Material | Any material or metal that has been manipulated before the operation must be carefully sterilized. | Custom-made polycarbonate templates. | In English: They have been successfully used intraoperatively to guide the intervention or postoperatively to educate other team members. |
| GME5 | Surgical tools and prototypes | Surgical instrument, instrument prototype, biomodels, prostheses, implants | ME38 | The objective of this article was to review the current applications of 3DP in modern surgical practice. | A electronic search was conducted in MEDLINE, EMBASE and PsycINFO for terms related to 3DP. These were then examined for their relevance and the practical applications of the technology in surgery. | Results: Initially, 488 articles were found, and these were finally reduced to 93 full-text articles. It was determined that there were three main areas in which technology is being used for printing: (1) anatomical models, (2) surgical instruments, and (3) implants and prostheses. | The different specialties are at different stages in the use of technology. The costs of implementing technology and the time it takes for printing are important factors to consider before widespread use. In the foreseeable future, it is an exciting and interesting technology with the ability to radically change healthcare and revolutionize modern surgery. | Costs and deadlines | significantly reduce the operation time and also improve accuracy. | Using 3D reconstructed images by CT scan. | The operations that use these guides have reduced operation times [9], but the preoperative phase of these operations takes longer as they require more image and data processing to produce the printed object. |
| GME5 | Surgical tools and prototypes | Surgical instrument, instrument prototype, biomodels, prostheses, implants | ME38 | The objective of this article was to review the current applications of 3DP in modern surgical practice. | A electronic search was conducted in MEDLINE, EMBASE and PsycINFO for terms related to 3DP. These were then examined for their relevance and the practical applications of the technology in surgery. | Results: Initially, 488 articles were found, and these were finally reduced to 93 full-text articles. It was determined that there were three main areas in which technology is being used for printing: (1) anatomical models, (2) surgical instruments, and (3) implants and prostheses. | The different specialties are at different stages in the use of technology. The costs of implementing technology and the time it takes for printing are important factors to consider before widespread use. In the foreseeable future, it is an exciting and interesting technology with the ability to radically change healthcare and revolutionize modern surgery. | Security | Any material or metal that has been manipulated before the operation must be carefully sterilized. | Custom-made polycarbonate templates. | In English: They have been successfully used intraoperatively to guide the intervention or postoperatively to educate other team members. |
| GME5 | Surgical tools and prototypes | Surgical instrument, instrument prototype, biomodels, prostheses, implants | ME38 | The objective of this article was to review the current applications of 3DP in modern surgical practice. | A electronic search was conducted in MEDLINE, EMBASE and PsycINFO for terms related to 3DP. These were then examined for their relevance and the practical applications of the technology in surgery. | Results: Initially, 488 articles were found, and these were finally reduced to 93 full-text articles. It was determined that there were three main areas in which technology is being used for printing: (1) anatomical models, (2) surgical instruments, and (3) implants and prostheses. | The different specialties are at different stages in the use of technology. The costs of implementing technology and the time it takes for printing are important factors to consider before widespread use. In the foreseeable future, it is an exciting and interesting technology with the ability to radically change healthcare and revolutionize modern surgery. | Legal Aspects | Any material or metal that has been manipulated before the operation must be carefully sterilized. | Custom-made polycarbonate templates. | In English: They have been successfully used intraoperatively to guide the intervention or postoperatively to educate other team members. |
| GME5 | Surgical tools and prototypes | Biological models, surgical guides, surgical simulators | ME53 | This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | Function | The insertion of pedicle screws for stabilization, reducing difficulty for less experienced surgeons, improving the accuracy and efficiency of perpendicular screw insertion. | The template guide typically consists of a part that matches the posterior vertebral surface and a part that traces a drilling path for screw insertion (e.g., 'screw guide cylinders') [46]. Once the template is printed, the screw insertion site and its ideal trajectory are preoperatively confirmed. This resulted in an increase of over 20% in the rate of acceptable screws (reduction in pedicle cortex perforations). | Combination of reverse engineering and modeling. Commercial software for this process includes MIMICS, Ziostation, UG Imageware, and Freeform [45]. The guide template is placed on the posterior vertebra to guide the placement of the pedicle screw. A high-speed drill is used to drill the pedicle screw along the trajectory indicated by the guide template [l3, l4, 47]. | |
| GME5 | Surgical tools and prototypes | Biological models, surgical guides, surgical simulators | ME53 | This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | Dimensions | that matches the posterior anatomy of the target vertebra | A 3DP model of the patient's target vertebra is generated from CT or MRI image data. A screw guide template is then reverse-engineered to match the posterior anatomy. | To generate a 3DP object, raw data from images is obtained, usually from computed tomography (CT) or magnetic resonance imaging (MRI), which is saved in the Data Imaging and Communications in Medicine (DICOM) file format. The data is processed using 3D modeling software to generate a computer-aided design (CAD) digital model. This three-dimensional excavation model is segmented into 2D layers, and the information is saved as a Standard Tessellation Language (STL) file. The segmentation step is performed to isolate the area or anatomical areas of interest and to generate a 'surface mesh' of that area (e.g., a single-target vertebra). The STL file, which is now readable by a 3D printer, is transferred to a printer for production [2]. Commercial software for this process includes MIMICS, Ziostation, UG Imageware, and Freeform [45]. | |
| GME5 | Surgical tools and prototypes | Biological models, surgical guides, surgical simulators | ME53 | This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | Customization | that matches the posterior anatomy of the target vertebra | A 3DP model of the patient's target vertebra is generated from CT or MRI image data. A screw guide template is then reverse-engineered to match the posterior anatomy. | To generate a 3DP object, raw data from images is obtained, usually from computed tomography (CT) or magnetic resonance imaging (MRI), which is saved in the Data Imaging and Communications in Medicine (DICOM) file format. The data is processed using 3D modeling software to generate a computer-aided design (CAD) digital model. This three-dimensional excavation model is segmented into 2D layers, and the information is saved as a Standard Tessellation Language (STL) file. The segmentation step is performed to isolate the area or anatomical areas of interest and to generate a 'surface mesh' of that area (e.g., a single-target vertebra). The STL file, which is now readable by a 3D printer, is transferred to a printer for production [2]. Commercial software for this process includes MIMICS, Ziostation, UG Imageware, and Freeform [45]. | |
| GME5 | Surgical tools and prototypes | Biological models, surgical guides, surgical simulators | ME53 | This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | Material | It should be economical, allow sterilization, and follow FDA guidelines. | SLS and SLA printing techniques are most commonly used to print these guide templates [l2, l3], although the use of FDM [48] has been reported. | Spinal products are approved by the FDA on a case-by-case basis [14]. [14] Garg B, Mehta N. Current status of 3D printing in spine surgery. J Clin Orthop Trauma 2018;9(3):218–25. https://doi.org/10.1016/j. jcot.2018.08.006. | |
| GME5 | Surgical tools and prototypes | Biological models, surgical guides, surgical simulators | ME53 | This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | Manufacturing and Assembly | It should be economical, allow sterilization, and follow FDA guidelines. | SLS and SLA printing techniques are most commonly used to print these guide templates [l2, l3], although the use of FDM [48] has been reported. | Spinal products are approved by the FDA on a case-by-case basis [14]. [14] Garg B, Mehta N. Current status of 3D printing in spine surgery. J Clin Orthop Trauma 2018;9(3):218–25. https://doi.org/10.1016/j. jcot.2018.08.006. | |
| GME5 | Surgical tools and prototypes | Biological models, surgical guides, surgical simulators | ME53 | This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | Costs and deadlines | It must have lower entry costs, and shorter times | A decrease of approximately 17 minutes in intraoperative time, and one-fifth of the cost with titanium guides if polymers are used [54]. | ||
| GME5 | Surgical tools and prototypes | Biological models, surgical guides, surgical simulators | ME53 | This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | Security | The template must be sterilized for intraoperative use. The 3DP template approach should have lower radiation exposure ref. [13-15]. Avoid waste during high-speed drilling. | A quadruple reduction in radiation frequency [51]. Or total reduction [52]. Total waste reduction by using titanium [54]. | If polymer-based material is used to build the 3DP guide template, high-speed intraoperative drilling can produce template waste. The use of Titaneo is sterile but increases the cost by 5 times [54]. | |
| GME5 | Surgical tools and prototypes | Biological models, surgical guides, surgical simulators | ME53 | This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | Legal Aspects | Spinal products are approved by the FDA on a case-by-case basis ref. [14] (Garg B, Mehta N. Current status of 3D printing in spine surgery. J Clin Orthop Trauma 2018;9(3):218–25. https://doi.org/10.1016/j.jcot.2018.08.006.) | It is based on literature search. [14] Garg B, Mehta N. Current status of 3D printing in spine surgery. J Clin Orthop Trauma 2018;9(3):218–25. https://doi.org/10.1016/j. jcot.2018.08.006 |
||
| GME5 | Surgical tools and prototypes | Surgical instrument | ME44 | To carry out a quick demonstration of additive manufacturing of surgical instruments in a field hospital setting. The objective of the demonstration was to show that, upon request, manufacturing in remote locations of a variety of sterile surgical instruments is a real possibility with current technology. | The following instruments were modeled in SolidWorks: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. A parametric 3D CAD model was created and modified for each instrument based on adaptations of stainless steel instruments. Key dimensions were identified and then defined as driving dimensions. The team chose to follow an evolutionary design approach due to time constraints. Designs of candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. The instruments were printed in ABSi-Ag, a silver-loaded ABS plastic resin under development by Stratasys. Samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. The instruments were initially tested by dissecting and suturing fried chickens obtained from a local grocery store. A 3D printer, a laptop, and a portable generator were set up next to the simulated field hospital store. A simulated laparotomy surgery was performed on a human surgical simulator 'Cut Suit', used by a volunteer model, using a kit of printed instruments. | In a period of less than three months, the team developed and demonstrated the ability to produce sterile surgical instruments in a field environment using a material extrusion 3D printer. The instruments were printed in a modified ABS plastic resin, extruded in layers to form the shapes of the instruments. The high processing temperatures of the ABS resin produced sterile parts directly from the printer. The rapid testing and iterations of the instrument designs resulted in a functional surgical kit that could be printed in a single construction on the 3D printer. At the end of the project, the 3D printer and the surgical instrument kit were demonstrated in a simulated field surgery. A team of military surgeons used the printed instruments to perform a laparotomy procedure on a model wearing a cut suit training simulator. | The successfully demonstrated manufacturing of a set of surgical instruments using 3D printing by material extrusion was shown using commercially available equipment and software programs. Biological tests of the printed samples in the laboratory demonstrated high sterility; however, further research on process control is necessary to achieve 100% sterility of all instruments. Additive manufacturing of surgical instrument equipment was demonstrated in a simulated field environment. Electrical power, digital files of the instruments, and ABS materials were all that was needed to print the instruments on demand. A catalog of thousands of open-source instrument designs, or even custom instruments, could be stored on digital media or accessed remotely from the internet for printing in the field. | Function | You must perform the functions of Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors made of conventional materials. | A 3D parametric CAD model was created for each instrument based on adaptations of commonly available stainless steel instruments. | The key dimensions were identified and then defined as driving dimensions for CAD geometry. The driving dimensions were modified to generate digital versions. The instruments were printed in ABS thermoplastic; therefore, it was necessary to modify these instrument designs to reproduce the mechanical performance of standard instruments (typically made of stainless steel). In particular, cutting instruments were designed with ABS bodies that accepted standard scalpel blades. The team chose to follow an evolutionary design approach due to time constraints. Candidate instrument designs were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME44 | To carry out a quick demonstration of additive manufacturing of surgical instruments in a field hospital setting. The objective of the demonstration was to show that, upon request, manufacturing in remote locations of a variety of sterile surgical instruments is a real possibility with current technology. | The following instruments were modeled in SolidWorks: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. A parametric 3D CAD model was created and modified for each instrument based on adaptations of stainless steel instruments. Key dimensions were identified and then defined as driving dimensions. The team chose to follow an evolutionary design approach due to time constraints. Designs of candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. The instruments were printed in ABSi-Ag, a silver-loaded ABS plastic resin under development by Stratasys. Samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. The instruments were initially tested by dissecting and suturing fried chickens obtained from a local grocery store. A 3D printer, a laptop, and a portable generator were set up next to the simulated field hospital store. A simulated laparotomy surgery was performed on a human surgical simulator 'Cut Suit', used by a volunteer model, using a kit of printed instruments. | In a period of less than three months, the team developed and demonstrated the ability to produce sterile surgical instruments in a field environment using a material extrusion 3D printer. The instruments were printed in a modified ABS plastic resin, extruded in layers to form the shapes of the instruments. The high processing temperatures of the ABS resin produced sterile parts directly from the printer. The rapid testing and iterations of the instrument designs resulted in a functional surgical kit that could be printed in a single construction on the 3D printer. At the end of the project, the 3D printer and the surgical instrument kit were demonstrated in a simulated field surgery. A team of military surgeons used the printed instruments to perform a laparotomy procedure on a model wearing a cut suit training simulator. | The successfully demonstrated manufacturing of a set of surgical instruments using 3D printing by material extrusion was shown using commercially available equipment and software programs. Biological tests of the printed samples in the laboratory demonstrated high sterility; however, further research on process control is necessary to achieve 100% sterility of all instruments. Additive manufacturing of surgical instrument equipment was demonstrated in a simulated field environment. Electrical power, digital files of the instruments, and ABS materials were all that was needed to print the instruments on demand. A catalog of thousands of open-source instrument designs, or even custom instruments, could be stored on digital media or accessed remotely from the internet for printing in the field. | Dimensions | It should be similar to conventionally manufactured instruments: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. | A 3D parametric CAD model was created for each instrument based on adaptations of commonly available stainless steel instruments. | The dimensions of driving were modified to generate digital versions. The designs of the candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome the identified deficiencies in terms of weight, balance, rigidity, and tactile control. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME44 | To carry out a quick demonstration of additive manufacturing of surgical instruments in a field hospital setting. The objective of the demonstration was to show that, upon request, manufacturing in remote locations of a variety of sterile surgical instruments is a real possibility with current technology. | The following instruments were modeled in SolidWorks: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. A parametric 3D CAD model was created and modified for each instrument based on adaptations of stainless steel instruments. Key dimensions were identified and then defined as driving dimensions. The team chose to follow an evolutionary design approach due to time constraints. Designs of candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. The instruments were printed in ABSi-Ag, a silver-loaded ABS plastic resin under development by Stratasys. Samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. The instruments were initially tested by dissecting and suturing fried chickens obtained from a local grocery store. A 3D printer, a laptop, and a portable generator were set up next to the simulated field hospital store. A simulated laparotomy surgery was performed on a human surgical simulator 'Cut Suit', used by a volunteer model, using a kit of printed instruments. | In a period of less than three months, the team developed and demonstrated the ability to produce sterile surgical instruments in a field environment using a material extrusion 3D printer. The instruments were printed in a modified ABS plastic resin, extruded in layers to form the shapes of the instruments. The high processing temperatures of the ABS resin produced sterile parts directly from the printer. The rapid testing and iterations of the instrument designs resulted in a functional surgical kit that could be printed in a single construction on the 3D printer. At the end of the project, the 3D printer and the surgical instrument kit were demonstrated in a simulated field surgery. A team of military surgeons used the printed instruments to perform a laparotomy procedure on a model wearing a cut suit training simulator. | The successfully demonstrated manufacturing of a set of surgical instruments using 3D printing by material extrusion was shown using commercially available equipment and software programs. Biological testing of the printed samples in the laboratory demonstrated high sterility; however, further research on process control is necessary to achieve 100% sterility of all instruments. Additive manufacturing of surgical instrument equipment was demonstrated in a simulated field environment. Electrical power, digital files of the instruments, and ABS materials were all that was needed to print the instruments on demand. A catalog of thousands of open-source instrument designs, or even custom instruments, could be stored on digital media or accessed remotely from the internet for printing in the field. | Strength | The translated value of the provided data Material debe ser resistente mecánicamente, ligero y balanceado in English is Material must be mechanically resistant, lightweight, and balanced. | Experimental tests are conducted on chicken for frying and surgical simulators. | The dissection and suturing was successfully performed on pieces of fried chicken, and then in a subsequent demonstration on a human surgical simulator. With the exception of the Metzenbaum scissors, all instruments worked properly for dissecting and suturing on the surgical simulator without failing. The scalpel blades were used to cut the scissor blades, but they often did not fit, so in many cases the tissue was not cut. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME44 | To carry out a quick demonstration of additive manufacturing of surgical instruments in a field hospital setting. The objective of the demonstration was to show that, upon request, manufacturing in remote locations of a variety of sterile surgical instruments is a real possibility with current technology. | The following instruments were modeled in SolidWorks: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. A parametric 3D CAD model was created and modified for each instrument based on adaptations of stainless steel instruments. Key dimensions were identified and then defined as driving dimensions. The team chose to follow an evolutionary design approach due to time constraints. Designs of candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. The instruments were printed in ABSi-Ag, a silver-loaded ABS plastic resin under development by Stratasys. Samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. The instruments were initially tested by dissecting and suturing fried chickens obtained from a local grocery store. A 3D printer, a laptop, and a portable generator were set up next to the simulated field hospital store. A simulated laparotomy surgery was performed on a human surgical simulator 'Cut Suit', used by a volunteer model, using a kit of printed instruments. | In a period of less than three months, the team developed and demonstrated the ability to produce sterile surgical instruments in a field environment using a material extrusion 3D printer. The instruments were printed in a modified ABS plastic resin, extruded in layers to form the shapes of the instruments. The high processing temperatures of the ABS resin produced sterile parts directly from the printer. The rapid testing and iterations of the instrument designs resulted in a functional surgical kit that could be printed in a single construction on the 3D printer. At the end of the project, the 3D printer and the surgical instrument kit were demonstrated in a simulated field surgery. A team of military surgeons used the printed instruments to perform a laparotomy procedure on a model wearing a cut suit training simulator. | The successfully demonstrated manufacturing of a set of surgical instruments using 3D printing by material extrusion was shown using commercially available equipment and software programs. Biological tests of the printed samples in the laboratory demonstrated high sterility; however, further research on process control is necessary to achieve 100% sterility of all instruments. Additive manufacturing of surgical instrument equipment was demonstrated in a simulated field environment. Electrical power, digital files of the instruments, and ABS materials were all that was needed to print the instruments on demand. A catalog of thousands of open-source instrument designs, or even custom instruments, could be stored on digital media or accessed remotely from the internet for printing in the field. | Material | The translated value of the provided data Material debe ser resistente mecánicamente, y ser estéril in English is Material must be mechanically resistant and sterile. | en ABSi-Ag, a silver-filled ABS plastic resin under development by Stratasys. The high processing temperatures of the ABS resin produced sterile parts, directly from the printer. | The high processing temperatures of ABS resin produced sterile parts, directly from the printer (300-311 C) in a controlled temperature environment (77 C). |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME44 | To carry out a quick demonstration of additive manufacturing of surgical instruments in a field hospital setting. The objective of the demonstration was to show that, upon request, manufacturing in remote locations of a variety of sterile surgical instruments is a real possibility with current technology. | The following instruments were modeled in SolidWorks: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. A parametric 3D CAD model was created and modified for each instrument based on adaptations of stainless steel instruments. Key dimensions were identified and then defined as driving dimensions. The team chose to follow an evolutionary design approach due to time constraints. Designs of candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. The instruments were printed in ABSi-Ag, a silver-loaded ABS plastic resin under development by Stratasys. Samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. The instruments were initially tested by dissecting and suturing fried chickens obtained from a local grocery store. A 3D printer, a laptop, and a portable generator were set up next to the simulated field hospital store. A simulated laparotomy surgery was performed on a human surgical simulator 'Cut Suit', used by a volunteer model, using a kit of printed instruments. | In a period of less than three months, the team developed and demonstrated the ability to produce sterile surgical instruments in a field environment using a material extrusion 3D printer. The instruments were printed in a modified ABS plastic resin, extruded in layers to form the shapes of the instruments. The high processing temperatures of the ABS resin produced sterile parts directly from the printer. The rapid testing and iterations of the instrument designs resulted in a functional surgical kit that could be printed in a single construction on the 3D printer. At the end of the project, the 3D printer and the surgical instrument kit were demonstrated in a simulated field surgery. A team of military surgeons used the printed instruments to perform a laparotomy procedure on a model wearing a cut suit training simulator. | The successfully demonstrated manufacturing of a set of surgical instruments using 3D printing by material extrusion was shown using commercially available equipment and software programs. Biological tests of the printed samples in the laboratory demonstrated high sterility; however, further research on process control is necessary to achieve 100% sterility of all instruments. Additive manufacturing of surgical instrument equipment was demonstrated in a simulated field environment. Electrical power, digital files of the instruments, and ABS materials were all that was needed to print the instruments on demand. A catalog of thousands of open-source instrument designs, or even custom instruments, could be stored on digital media or accessed remotely from the internet for printing in the field. | Manufacturing and Assembly | It should be economical compared to traditional methods and fast. | FDM, Dimension uPrint Plus SE (Stratasys, Eden Prairie, MN). | To form the pieces, the 3D printer deposited filaments of melted thermoplastic material (300-311 C) in a controlled temperature environment (77 C), where the material solidified quickly. The material was deposited on a flat platform of 203 square mm in a buildup of layers. First, multiple layers of support material were deposited to form a fixing substrate on the platform. Then, layers of 0.254 mm thick structural ABS were deposited from the bottom of the parts upwards. The instruments were printed in ABSi-Ag, an ABS plastic resin with silver filler. The support structures were printed in Stratasys' P400-SR and SR30-XL materials; these materials were designed to provide structural support for the part during manufacturing while minimizing interface joints with the ABS for easy removal. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME44 | To carry out a quick demonstration of additive manufacturing of surgical instruments in a field hospital setting. The objective of the demonstration was to show that, upon request, manufacturing in remote locations of a variety of sterile surgical instruments is a real possibility with current technology. | The following instruments were modeled in SolidWorks: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. A parametric 3D CAD model was created and modified for each instrument based on adaptations of stainless steel instruments. Key dimensions were identified and then defined as driving dimensions. The team chose to follow an evolutionary design approach due to time constraints. Designs of candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. The instruments were printed in ABSi-Ag, a silver-loaded ABS plastic resin under development by Stratasys. Samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. The instruments were initially tested by dissecting and suturing fried chickens obtained from a local grocery store. A 3D printer, a laptop, and a portable generator were set up next to the simulated field hospital store. A simulated laparotomy surgery was performed on a human surgical simulator 'Cut Suit', used by a volunteer model, using a kit of printed instruments. | In a period of less than three months, the team developed and demonstrated the ability to produce sterile surgical instruments in a field environment using a material extrusion 3D printer. The instruments were printed in a modified ABS plastic resin, extruded in layers to form the shapes of the instruments. The high processing temperatures of the ABS resin produced sterile parts directly from the printer. The rapid testing and iterations of the instrument designs resulted in a functional surgical kit that could be printed in a single construction on the 3D printer. At the end of the project, the 3D printer and the surgical instrument kit were demonstrated in a simulated field surgery. A team of military surgeons used the printed instruments to perform a laparotomy procedure on a model wearing a cut suit training simulator. | The successfully demonstrated manufacturing of a set of surgical instruments using 3D printing by material extrusion was shown using commercially available equipment and software programs. Biological tests of the printed samples in the laboratory demonstrated high sterility; however, further research on process control is necessary to achieve 100% sterility of all instruments. Additive manufacturing of surgical instrument equipment was demonstrated in a simulated field environment. Electrical power, digital files of the instruments, and ABS materials were all that was needed to print the instruments on demand. A catalog of thousands of open-source instrument designs, or even custom instruments, could be stored on digital media or accessed remotely from the internet for printing in the field. | Costs and deadlines | The translated value of Los tiempos de impresión deben ser pequeños in English is Printing times should be small. | The printing time took 1 to 3 hours per instrument, and the complete kit less than 6 hours. | |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME44 | To carry out a quick demonstration of additive manufacturing of surgical instruments in a field hospital setting. The objective of the demonstration was to show that, upon request, manufacturing in remote locations of a variety of sterile surgical instruments is a real possibility with current technology. | The following instruments were modeled in SolidWorks: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. A parametric 3D CAD model was created and modified for each instrument based on adaptations of stainless steel instruments. Key dimensions were identified and then defined as driving dimensions. The team chose to follow an evolutionary design approach due to time constraints. Designs of candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. The instruments were printed in ABSi-Ag, a silver-loaded ABS plastic resin under development by Stratasys. Samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. The instruments were initially tested by dissecting and suturing fried chickens obtained from a local grocery store. A 3D printer, a laptop, and a portable generator were set up next to the simulated field hospital store. A simulated laparotomy surgery was performed on a human surgical simulator 'Cut Suit', used by a volunteer model, using a kit of printed instruments. | In a period of less than three months, the team developed and demonstrated the ability to produce sterile surgical instruments in a field environment using a material extrusion 3D printer. The instruments were printed in a modified ABS plastic resin, extruded in layers to form the shapes of the instruments. The high processing temperatures of the ABS resin produced sterile parts directly from the printer. The rapid testing and iterations of the instrument designs resulted in a functional surgical kit that could be printed in a single construction on the 3D printer. At the end of the project, the 3D printer and the surgical instrument kit were demonstrated in a simulated field surgery. A team of military surgeons used the printed instruments to perform a laparotomy procedure on a model wearing a cut suit training simulator. | The successfully demonstrated manufacturing of a set of surgical instruments using 3D printing by material extrusion was shown using commercially available equipment and software programs. Biological tests of the printed samples in the laboratory demonstrated high sterility; however, further research on process control is necessary to achieve 100% sterility of all instruments. Additive manufacturing of surgical instrument equipment was demonstrated in a simulated field environment. Electrical power, digital files of the instruments, and ABS materials were all that was needed to print the instruments on demand. A catalog of thousands of open-source instrument designs, or even custom instruments, could be stored on digital media or accessed remotely from the internet for printing in the field. | Security | The translated value of El material debe ser estéril in English is The material must be sterile. | The high processing temperatures of ABS resin produced sterile parts, directly from the printer. | The sample instruments were printed on an identical device at the Naval Postgraduate Dental School (NPDS) and the Edgewood Chemical Biological Center (ECBC). The sterility of the printed instruments at both locations was evaluated at the ECBC. The samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. Turbidity was observed to evaluate bacterial growth; lack of turbidity in the culture medium indicated a lack of microbial load in the instrument samples. Once the pieces were partially separated from the support substrate, they could be manually removed with gloved fingers. It was determined that 90% of the sampled instruments, directly from the 3D printer, were sterile. However, further research on process control is necessary to achieve 100% sterility of all instruments. |
| GME5 | Surgical tools and prototypes | Surgical instrument | ME44 | To carry out a quick demonstration of additive manufacturing of surgical instruments in a field hospital setting. The objective of the demonstration was to show that, upon request, manufacturing in remote locations of a variety of sterile surgical instruments is a real possibility with current technology. | The following instruments were modeled in SolidWorks: Kelly Hemostat, needle holder, tissue forceps, retractor, scalpel handle, and Metzenbaum scissors. A parametric 3D CAD model was created and modified for each instrument based on adaptations of stainless steel instruments. Key dimensions were identified and then defined as driving dimensions. The team chose to follow an evolutionary design approach due to time constraints. Designs of candidate instruments were digitally created, manufactured, evaluated, and modified as necessary to overcome identified deficiencies in terms of weight, balance, stiffness, and tactile control. The instruments were printed in ABSi-Ag, a silver-loaded ABS plastic resin under development by Stratasys. Samples were tested for sterility by immersing them in 20 mL of autoclave-promoted microbial growth medium, tryptic soy broth. The devices were cultured overnight at 37°C on an orbital shaker (at 170 rpm) to promote microbial growth. The instruments were initially tested by dissecting and suturing fried chickens obtained from a local grocery store. A 3D printer, a laptop, and a portable generator were set up next to the simulated field hospital store. A simulated laparotomy surgery was performed on a human surgical simulator 'Cut Suit', used by a volunteer model, using a kit of printed instruments. | In a period of less than three months, the team developed and demonstrated the ability to produce sterile surgical instruments in a field environment using a material extrusion 3D printer. The instruments were printed in a modified ABS plastic resin, extruded in layers to form the shapes of the instruments. The high processing temperatures of the ABS resin produced sterile parts directly from the printer. The rapid testing and iterations of the instrument designs resulted in a functional surgical kit that could be printed in a single construction on the 3D printer. At the end of the project, the 3D printer and the surgical instrument kit were demonstrated in a simulated field surgery. A team of military surgeons used the printed instruments to perform a laparotomy procedure on a model wearing a cut suit training simulator. | The successfully demonstrated manufacturing of a set of surgical instruments using 3D printing by material extrusion was shown using commercially available equipment and software programs. Biological tests of the printed samples in the laboratory demonstrated high sterility; however, further research on process control is necessary to achieve 100% sterility of all instruments. Additive manufacturing of surgical instrument equipment was demonstrated in a simulated field environment. Electrical power, digital files of the instruments, and ABS materials were all that was needed to print the instruments on demand. A catalog of thousands of open-source instrument designs, or even custom instruments, could be stored on digital media or accessed remotely from the internet for printing in the field. | Legal Aspects | The translated value of El material debe ser estéril in English is The material must be sterile. | The high processing temperatures of ABS resin produced sterile parts, directly from the printer (300-311 C) in a controlled temperature environment (77 C). | Once the pieces were partially separated from the support substrate, they could be manually removed with gloved fingers. It was determined that 90% of the sampled instruments, directly from the 3D printer, were sterile. However, further research on process control is necessary to achieve 100% sterility of all instruments. |
| GME5 | Surgical tools and prototypes | surgical instrument, instrument prototype | ME74 | This concept of custom surgical instruments was investigated as a pilot demonstration of mass customization and on-demand manufacturing by a team from the Naval Postgraduate Dental School (Bethesda, MD), Stratasys (Eden Prairie, MN), and the Defense Advanced Research Projects Agency (Arlington, VA) Service Chiefs Fellowship Program. Spring devices for custom orthodontic brackets and customized pivot pliers were digitally designed, manufactured, and evaluated using a surgical simulator. | The digital models of a spring clamp and a central pivot clamp were created in the SolidWorks 2012 computer-aided design (CAD) software. The models were parameterized with key dimensions (arm lengths, finger loop positions, etc.). Custom designs were generated simply by changing the values of the key dimensions within the CAD application. The instruments were sized to fit the hands of a clinician and tailored to express personal preferences and preferred technique. It was necessary to adapt the designs to accommodate the characteristics of currently available materials; this involved thickening the cross-sectional area of the clamp arms and redesigning the simple pivot hinges to provide the appropriate mechanical strength and rigidity. A Stratasys ABSi-Ag development material was used to print examples of the instruments. This material is currently being developed as a potentially biocompatible and bacteriostatic material for medical applications. Custom tissue forceps and a needle driver were designed, manufactured, and tested according to the physician's personal specifications. A subjective evaluation was performed by performing surgical procedures and suturing an incision on a surgical simulator of a cut suit. | A one-piece, spring-style tissue forceps design was created in CAD. The design was custom-made to fit the hand of a clinician, and later adapted in stiffness to provide the desired sensation with palm pressure. Interlocking triangular teeth were added to the tip of the instrument, according to the clinician's preference. Simple grip and hold tests were performed to evaluate the design; the information provided by the clinician led to further modifications. The stiffness of the arms, the length of the arms, and the angle of the arms in closure were modified at the request of the clinician. The change in the material deposition pattern in the material extrusion process affected the instrument's sensation as reported by the clinician. A two-piece hinge pivot forceps design was also created in CAD. A standard pivoting arm design, common to hemostats, was modified for production in ABS plastic. A 'T'-shaped pivot key and a tapered slot joined the arms. Handling dimensions included arm length, arm section, jaw length, jaw section, finger loop diameter, and position. The clinician expressed a desire for an instrument that could be picked up, manipulated, and dropped from a gloved hand with minimal manual articulation. The serrated jaws were sized for dissection and holding tasks. A closing zipper tooth was added to the back of the arm handles for closure; the zipper was operated by palm pressure. A basic surgery kit was printed on the FDM device, including tissue forceps, hemostat, and needle holders; the printing time was just over 6 hours. Successful laparotomy, ligation, splenectomy, and suturing were performed in the cutting suit using the custom instruments. | The instruments of spring and pivot pliers were designed according to the specifications of a clinical test, adapted to the dimensions of the hand, personal preference for the instrument's feel, and preferred surgical technique. The instruments were 3D printed on demand using a material extrusion 3D printer and then successfully used to complete surgical procedures on a realistic human simulator. With the development of new biocompatible materials for additive manufacturing, it will be possible to 3D print custom surgical instruments on demand. With design and manufacturing cycles of just a few hours, it will be feasible to quickly develop new instruments tailored to new surgical techniques and procedures. | - | - | - | - |
| GME5 | Surgical tools and prototypes | surgical instrument, instrument prototype | ME37 Pro9 | The treatment of combat casualties in the operational zone presents a set of challenges, including the logistical challenge of providing military surgeons with sterile surgical materials. 3D printing can offer a solution to overcome the logistical challenge by using resistant, durable, and biocompatible thermoplastic resins that can be molded into any shape, through additive manufacturing, to produce sterile surgical instruments on demand, in deployed medical facilities outside the national territory. | Select the most appropriate general surgical instruments for this project: It is based on the general instruments used in orthopedic surgery requested in more than 80% of orthopedic and traumatology surgeries. • Development of the necessary software to create the plans for each of the instruments in the language of the 3D printer, to create the plans for each instrument to be printed. The software has the extension .stl • Printing of these instruments for non-real manipulation and evaluation of possible errors in the design: By cyclic loading on each of the instruments made, we seek material fatigue until its rupture, thus fixing the number of cycles it can withstand. (We consider a cycle to be the maximum manual load that we are capable of exerting on the separator for at least 5 minutes, thus simulating the moments of greatest stress on the instrument in the operating room). • The last step would include sending, along with the rest of the healthcare step, a 3D printer, the STL files of each instrument, and the necessary PLA coils for printing. | - | - | - | - | ||
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts. Tolerances were verified after sterilization, as well as the fit with the printed bone model and the straightening of the bone model. A program was developed to automate the design of the wedge through 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Wedge material | It must withstand impact loads during surgical procedures without failure, and it must withstand sterilization temperature without deformations that modify the angle of surgical correction. This implies high toughness, high melting temperature, high heat deformation resistance, and low cost. | Computer | Evaluation by weighted averages, Numerical modeling by finite elements and experimentation |
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts, tolerances were verified after sterilization, and the fit with the printed bone model to be corrected as well as the straightening of the bone model were verified. A program was developed to automate the design of the Wedge by 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original one, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Wedge geometry | It must correspond to the location of the bone defect, the type of osteotomy, and the correction angle, extracted from a tomography or, failing that, from a radiograph. | Closing osteotomy, 65x9x9mm, 8° | Selection and calculation based on the guide Varus knee osteotomies: when and how to perform them [44] by Traumatologist Pablo Crespo Hernández, and the patient's tomography. |
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts. Tolerances were verified after sterilization, as well as the fit with the printed bone model and the straightening of the bone model. A program was developed to automate the design of the wedge through 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Wedge Temperature | The wedge is not an implant, but being in contact with bone tissue during surgery, it must withstand the sterilization process temperatures prior to surgery. By withstand, it means not to deteriorate mechanically or dimensionally. | 122-130°C for 20 to 30 minutes | Bibliographic reference sterilization process with autoclave, and autoclave technical specifications. |
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts, tolerances were verified after sterilization, and the fit with the printed bone model to be corrected as well as the straightening of the bone model were verified. A program was developed to automate the design of the Wedge by 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Impact load on wedge | The implant mold must withstand impact loads during the introduction of the wedge into bone tissue, and straightening, which is done manually using a hammer. | Sure, I can help you with that. Here is the translation of the provided data 2-106 N into English: 2-106 N translated into English is 2-106 N. I have removed the quotation marks from the translated value as requested. Let me know if there is anything else I can assist you with. | Measurement of load applied by impact with a hammer using a digital scale, indirect measurement through measurement of velocity and impact time with visual software, and numerical modeling of finite element impact load. |
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts, tolerances were verified after sterilization, and the fit with the printed bone model to be corrected as well as the straightening of the bone model were verified. A program was developed to automate the design of the Wedge by 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original one, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Material of Bone Prototype Models | Low printing temperature, Biodegradable, Easy access and low cost, Low resistance, low expansion coefficient. These materials will be used for the manufacture of bone tissue for correction, and verification of tolerances/adjustments of the correction with a wedge. It does not require special qualities of resistance to loads or temperature, since they will not be used directly during the procedure, the most desirable quality is dimensional accuracy to replicate the patient's bone members. | ABS | As the bones to be intervened will also be printed, but they must not meet the criteria of the wedge, the material that follows in the positions will be selected, which is ABS (3.9/5), as it is one of the most accessible and multifunctional materials on the table. |
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts, tolerances were verified after sterilization, and the fit with the printed bone model to be corrected as well as the straightening of the bone model were verified. A program was developed to automate the design of the Wedge by 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original one, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Geometry of Bone Prototype Models | The bone tissue must be of the specific shape and size customized for the patient (bone tomography) | Computed Tomography of Patient / Dicom Postprocessing in meshmixer, Solidwork, simplify3d | Recommendation Director, Advisor |
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts, tolerances were verified after sterilization, and the fit with the printed bone model to be corrected as well as the straightening of the bone model were verified. A program was developed to automate the design of the Wedge by 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original one, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Legal and Biocompatibility | The ISO 5832-1 standard of 2007 [28] establishes metallic materials for the manufacture of surgical implants, in order to prevent adverse reactions in the body and ensure the most acceptable biological response. It also specifies the methods and procedures for testing these materials. The same applies to implants made of high molecular weight polyethylene, whose performance tests and manufacturing procedures, as well as percentages of other plastics in the implant, are found in the ISO 5832-4 standard of 2011. | Computer | PC is a material used for implants that meets all legal requirements (Medical Advisor Recommendation) |
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts, tolerances were verified after sterilization, and the fit with the printed bone model to be corrected as well as the straightening of the bone model were verified. A program was developed to automate the design of the Wedge by 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original one, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Sterilization | With regard to Colombian legislation, for sterilization and cleaning procedures of surgical instruments, it is established through Resolution 2183 of 2004 from the Ministry of Health and Social Protection [30] the mandatory use of the Good Sterilization Practices Manual for Health Service Providers. Internationally, sterilization processes are governed by the Laboratory Biosafety Manual, published by the WHO, which specifies how laboratory tests on surgical instruments should be performed and their cleaning execution. Internationally, the Sterilization Manual for Health Centers also stands out, summarizing cleaning products, hand washing, and disinfection, as well as addressing topics on health education. | Sterilization by autoclave (110-130°C for 3 to 10 minutes), and/or hydrogen peroxide plasma (45°C for 45 to 80 minutes) | The polycarbonate complies with the biocompatibility standards [53] established by ISO 10993-1 [54] and USP Class VI [55], which verify the response of fluids and body tissues to the entry of plastic materials into the body. (for example, pipes in renal dialysis equipment or even instruments for heart surgeries [53]) |
| GME5 | Surgical tools and prototypes | Surgical guides | ME83 | • Generate the model of the wedge and specific bones of a patient, using tomographies, specialized software, and under the demand of the specialist. • Recreate the conditions to which the wedge will be subjected during surgery in order to select suitable materials for its manufacture through temperature and impact simulations. • Build a wedge for use in preoperative processes for a medical group during surgery, manufactured through the implementation of computer tools and 3D printing (FDM). • Perform functional tests on the wedge to verify that it meets the requirements and needs of the patient and specialists through impact and sterilization temperature tests. | From the lower bone tomography and specialized medical guide, a wedge geometry is created according to the correction to be made. The selection of materials is done using a weighted average matrix and the final material is defined by verifying wedge stresses and deformations during surgery impact load and autoclave sterilization using finite element simulation. Subsequently, the wedge was fabricated in PC and tested with real impacts, tolerances were verified after sterilization, and the fit with the printed bone model to be corrected as well as the straightening of the bone model were verified. A program was developed to automate the design of the Wedge by 3D printing. | The wedge passed the impact tests it was subjected to without any damage, after the sterilization test in the autoclave, the thermal distortion produced dimensional and geometric deviations within the allowed tolerances, the bone model straightening test was successful when comparing the corrected bone model with the original one, the wedge did not suffer any damage as predicted by the numerical simulations. The program to automatically design the wedge turned out to be feasible due to the simplicity of the wedge, with the previous procedure for deducing the angle and size of the wedge being more complex. A program was developed to automatically design the printed wedge. Project results: 1 Registered software, 1 Elaborated manuscript, 1 wedge prototype, 2 bone tissue prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture a wedge for lower limb osteotomy, the material used for the wedge was PC, the wedge was tested under impact load simulating the loads during surgery, and was subjected to an autoclave test to check for dimensional distortions, but after successful tests which predicted the numerical simulations by finite elements, the correction in a bone model was also successful, demonstrating the feasibility of using 3D printing by FFF to design, manufacture and intervene on the patient. A program was developed to systematize the design of the wedge and reduce design times, the feasibility of this program was demonstrated due to the simplicity of the wedge, where the most complex part is the calculation procedure of the dimensions and correction angle of the wedge, whose guide was incorporated into the program. | Automated design process of the wedge. | Develop a program that, based on the patient's geometry (tomography or radiography) and the correction angle calculation guide, generates a wedge for osteotomy. | Computer tool created in SolidWorks program with macros tool, programmed in Visual Basic | Recommendation Director, Advisor |
| GME5 | Surgical tools and prototypes | prototype instrument | ME85 | The general objective is the design and manufacture of a prototype of a self-supported abdominal separator for abdominoplasty. | • Conceptually design a self-supported mechanism to separate the abdominal walls without compromising the patient's physical integrity, facilitating the development of the procedure and not requiring prolonged effort from the instrumenters or the surgeon. • Design the prototype in detail by calculating mechanical and joining elements, and selecting them through catalogs, in order to verify the proposed design through load, displacement, deformation, and collision simulations using CAD software SolidWorks®. • Manufacture the self-supported abdominal separator prototype using fused deposition modeling (FDM) to subsequently perform operating tests. • Perform operating tests on the abdominal separator to verify the ease of manipulation and stability of the prototype, in order to ensure that there is no tissue necrosis in the patient and reduce the effort required by the surgical staff. • Evaluate the manufacturing costs of the separator with standardized materials for abdominoplasty surgery and compare them with existing ones, in order to verify its profitability. | - | - | - | - | ||
| GME5 | Surgical tools and prototypes | Surgical guides | ME52 | Computer-assisted planning and patient-specific 3D printed instrumental guides provide excellent assistance for these two steps, respectively in correcting post-traumatic limb deformity (osteotomy). | At the end, the corrective closing wedge osteotomy was chosen. The authors believed that it was the one that could best improve the overall alignment of the lower limb, relieve pain, and preserve the remaining range of motion. Unnecessary complications of RTC could also be avoided. In view of the anticipated difficulties (fracture with previous implants), computer-assisted 3D planning was used with the help of computed tomography. The malformed femur (accident) was virtually reconstructed and the deformity was measured. Computed tomography images and 3D reconstructions of the contralateral limb were also obtained as mirror reference. From the surgeons' point of view, the technique required in the planning of the corrective osteotomy was similar to the traditional method. Deformities in the coronal and sagittal planes were corrected by two osteotomy cuts, while rotational deformity was corrected by rotation during the final opposition. Data including the apex, angle of the planned cuts, planned hinge, and translation were entered into the computer, which provided real-time virtual images of the corrected femur. These images allowed for easy adjustment of the planning. The planned cuts that generated the virtual image that most closely resembled the ideal alignment based on the contralateral limb image were the chosen cuts. The subsequent fixation with a locking plate was planned based on the corrected alignment of the femur (Figure 5). 3D models of the distal femur (both pre-osteotomy and post-osteotomy), the marking guide, and custom-made osteotomy guide models for the patient were 3D printed (Figure 6). The patient was placed under general anesthesia and positioned supine on a radiolucent drop table. Previous implants that would hinder subsequent procedures were removed and a lateral approach to the distal femur was performed. The 3D printed guide for bone resection and osteotomy was sequentially and temporarily fixed to the distal femur with multiple Kirschner wires (Kirschner wires). Subsequently, screw holes for definitive fixation were made and the bone was cut accordingly (Figure 7). The alignment of the femur was corrected by opposing the planes of the osteotomy, which was then fixed with a locking compression plate with pre-drilled screw holes through the guide before the osteotomy. Therefore, the cut bone surfaces would oppose each other and the alignment would be corrected according to preoperative planning by applying the plate and tightening the screws. During the application of the locking compression plate, it was observed that interfragmentary compression could not be achieved using the previously drilled screw holes, so a proximal screw was inserted into an eccentric hole to achieve axial compression at the site of the osteotomy. | Surgical uncertainty, subjective judgment, radiation exposure, and operation duration were reduced compared to traditional methods. Alignment was confirmed radiologically and clinically, after which autogenous and synthetic bone grafts were added as supplements. The operation proceeded smoothly and largely as planned, without surgery-related complications. *The patient was allowed to walk on the ground immediately after the operation for 4 weeks, followed by another 4 weeks of partial weight-bearing and then full weight-bearing. *At 7 months post-operation, the patient enjoyed a stable and pain-free left knee, with a static range of motion (10°-20°, same as preoperative range) and strength (4/5 according to the Medical Council Research scale, same as preoperative strength). They resumed walking without assistance and returned to their previous work. *Radiologically, progressive union and consolidation were observed in the osteotomy area (Figures S and 9). An 18° correction in varus malalignment and a 14° correction in flexion were observed on the X-rays. This alignment matched the preoperative planning and remained consistent throughout the union process. Due to the correction of alignment in the coronal and sagittal planes, the shortening of the left lower limb improved with a 1.5 cm increase in length. | In the past, corrective osteotomy planning was mainly based on 2D radiographs with the help of marking markers on transparencies. It was a difficult task even in the most experienced hands, especially in cases with multiplanar deformities due to the complexity of the correction. With these limiting factors, overall accuracy was often unsatisfactory. In summary, preoperative planning, intraoperative execution, postoperative rehabilitation, and recovery were smooth and the functional outcome was satisfactory. During the operation, surgeons encountered some difficulties in manipulating the two ends of the bone after osteotomies. In retrospect, we think that an osteotomy guide with better design or a greater number of guides could provide temporary stability and refine the operative steps. Pre-drilled locking screw holes also hindered the application of interfragmentary compression. We believe that this experience will help us fine-tune the planning in subsequent cases. Traditional alternatives for deformity correction, including the Ilizarov technique and the use of the Taylor spatial frame, remain irreplaceable. They provide an alternative for gradual correction of the deformity, especially in severe deformities where soft tissue tolerance is a major concern. In addition to recent advances in preoperative planning, intraoperative execution can now be assisted by computer-assisted surgery, which is often used in total knee arthroplasty and tumor resection. Theoretically, computer-assisted surgery improves the accuracy of bone cuts and shortens the operation time. With the popularization of 3D printing technology in orthopedic surgery, more precise and accurate operations are expected. 3D-printed patient-specific instrumentation (PSI) has great versatility of application. In our case, the proper application of the PSI guide was facilitated by the identification of a well-formed osteophyte, while stable fixation was achieved using multiple Kirschner wires. In cases where a reliable bony landmark is not available, computer navigation could be helpful. | - | - | - | - |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Implant Material | The implant material will be a special resin for bone applications called PMMA, which is preferred over titanium for small implants. It is manufactured by mixing resin (main component and catalyst) generating an exothermic reaction, during which it must be molded to take its final shape. | PMMA (High Viscosity Acrylic Bone Cement BAUMER 1099) | Medical Advisor Recommendation |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Implant Geometry | The implant must be of the specific shape and size of the bone defect based on personalized measurements of the patient (bone tomography) | Computed Tomography of Patient / Dicom Postprocessing in meshmixer, Solidwork, simplify3d | Medical Advisor Recommendation |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Mold Temperature | The implant mold must withstand the exothermic reaction temperatures during PMMA resin pouring after mixing and during molding. By withstand, it refers to not deteriorating mechanically or dimensionally. | 95-110 °C | Direct measurement with infrared thermometer and manufacturer specifications PMMA. |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Compression Load of the Mold | The implant mold must withstand compression loads during molding, or manual mold closure, which essentially subjects the mold faces to manual compression loads. | Rigid Mold: approximately 10kg, 100N / Flexible Mold: approximately 2.5kg, 25N | Measurement of compression applied to a mold, using a digital scale. |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Geometry of the Mold | The mold must have a geometry that facilitates the pouring of the resin and its distribution, the shape must facilitate the application of compression load during molding, and facilitate the venting, opening or ejection of the implant out of the mold. | Rigid mold: volume 50x60x60mm, wall 5mm, hole 6mm / Flexible mold: 60mm diameter x 45mm height, slot 1mm. | Finite element modeling to verify safety factors and deformation under load and molding temperature. |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Mold Material | The mold must have a low coefficient of thermal expansion, high stiffness and strength modules, which implies that at high temperatures the mold deforms little and withstands the imposed loads without failure; the working temperature of the mold must be higher than the exothermic reaction temperature of PMMA; the material cost must be as low as possible; the manufacturing times must be as short as possible. | Rigid Mold: ABS, PC / Flexible Mold: Mold Star 16 Fast | Evaluation by weighted averages, and experimentation |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Material of implant models and skull | Low printing temperature, Biodegradable, Easy access and low cost, Low resistance, low expansion coefficient. These materials will be used for the manufacture of the Mold, and verification of tolerances/adjustments of the manufactured implant. It does not require special qualities of resistance to loads or temperature, since they will not be used directly during molding (exothermic reaction), the most desirable quality is dimensional accuracy to replicate the patient's bone members. | PLA | Recommendation Director, Advisor |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Geometry of Implant Models and Skull | The implant and bone tissue must be of the specific shape and size customized for the patient (bone tomography) | Computed Tomography of Patient / Dicom Postprocessing in meshmixer, Solidwork, simplify3d | Recommendation Director, Advisor |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Legal and Biocompatibility | The ISO 5832-1 standard of 2007 [28] establishes the metallic materials for the manufacture of surgical implants, in order to prevent adverse reactions in the body and to ensure the most acceptable biological response. It also specifies the methods and procedures for testing these materials. | PMMA (High Viscosity Acrylic Bone Cement BAUMER 1099) | PMMA is a material used for implants that meets all legal requirements (Medical Advisor Recommendation) |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | - | The same applies to implants made of high molecular weight polyethylene, whose performance tests and manufacturing procedures, as well as percentages of other plastics in the implant, are specified in ISO 5832-4:2011 [29]. | - | - |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Sterilization | As a master translator, I have translated the provided data into English. Here is the With regard to Colombian legislation, for sterilization and cleaning procedures of surgical instruments, it is established through Resolution 2183 of 2004 from the Ministry of Health and Social Protection [30] the mandatory use of the Good Sterilization Practices Manual for Health Service Providers. Internationally, sterilization processes are governed by the Laboratory Biosafety Manual [31], published by the WHO, which specifies how laboratory tests on surgical instruments should be performed and the execution of their cleaning. | Sterilization by autoclave (110-130°C for 3 to 10 minutes), and/or hydrogen peroxide plasma (45°C for 45 to 80 minutes) | PMMA is a material used for implants that meets all sterilization requirements. As for possible sterilization of molds, the working temperatures of these can withstand autoclave temperature (PC 110-130°C, Mold Star 16 Fast 232°C), according to technical specifications and previous experience of the director and advisor (Director's Recommendation, Advisor). |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | - | In the international field, the Manual de Esterilización para Centros de Salud [32] also stands out, which summarizes cleaning products, hand washing, and disinfection, as well as addressing topics on health education. | - | - |
| GME6 | Implants | Bone implant | ME84 | • Select the specific case study with its respective computed tomography images to generate the virtual model in CAD/CAM plans. • Design the prototype of the bone mold to be treated, taking into account body symmetry and the main loads and stresses to which it is functionally subjected. • Manufacture the prototype mold through 3D printing, then evaluate its performance and make it an iterative process until a better result is achieved. • Design a computer tool that facilitates the creation of the mold by providing the piece with personalized measurements. | From the skull tomography, a virtual model of the implant is created and subtracted. From the virtual model of the implant and its 3D printed model (FFF), rigid molds are designed and manufactured using 3D printing, and flexible molds are manufactured using flexible silicone molding. The selection of materials is done using a weighted average matrix, and the final size is defined by verifying stresses and deformations of the molds during implant manufacturing using finite element simulation. Subsequently, the implants were fabricated in PMMA and tested for fit with the printed bone model of the corrected skull. Software was developed to automate the design of rigid molds through 3D printing. | The implant made from the rigid mold had problems in demolding. The implant made from the flexible mold did not present any inconveniences in demolding, dimensionally complying with the tolerances and adjustments corresponding to the model of the corrected skull. The mold did not suffer any damage as predicted by the numerical simulations. A program was developed to automatically design the printed rigid mold based on the external measurements and the digital model of the implant. The following summarizes the products obtained from the project: 1 Registered Software, 1 Elaborated Manuscript, 2 mold prototypes, 2 implant prototypes. | Digital design was used based on computed tomography, finite element simulation, and material selection by weighted averages, to design and manufacture molds for PMMA bone implants. 3D printing and silicone molding were used for manufacturing and subsequent testing, with the mixed method of 3D printed model with flexible silicone mold being more dimensionally accurate and easy to demold. A program was developed to systematize the design of the rigid mold and reduce design times, once the implant model was generated, speed in redesign was demonstrated through the program. | Automated Mold Design Process | Develop a program that, based on the implant's geometry and the user-specified external dimensions of the mold, generates implant molds, with a view to verifying thermal deformations and mechanical strength of the design. | Computer tool created in SolidWorks program with macros tool, programmed in Visual Basic | Recommendation Director, Advisor |
| GME6 | Implants | Bone implant | ME54, ME55 | illustrate the capabilities of AM in the design and manufacturing of medical implants. | Literature review | In the biomedical field, many efforts are being made to develop new materials and/or improve the properties of materials (metals and polymers) for biomedical applications. In addition, the evolution of manufacturing processes, coatings, and surface implant modification are notable areas of work. Nowadays, metals are the best choice for long-term load-bearing prostheses. For this reason, the development of new metallic biomaterials for long-term prostheses and the adaptation of manufacturing processes to achieve the best possible properties is a matter of great interest. * Improvement of biocompatibility and modulus. * A ray strategy called MultiBeam has been launched, which allows for the production of finer details and a better surface finish. * New 3D CAD tools will allow for more freedom in design. Most commonly used commercial 3D CAD tools are designed for conventional manufacturing processes and do not easily allow for the design of new concepts with complex geometries, such as scaffolds, fractals, or bionic features. * Expert systems for process planning automation. * Specific CAE tools for scanner-to-part reconstruction, automatic guidance in implant design in STL format, assisted automated topological optimization of network structures, etc. | The technology EBM has proven to be a very powerful tool for the manufacturing of high value-added products. At the same time, EBM is a relatively recent manufacturing technology and has much more room for improvement. For both reasons, there are different R&D attempts being carried out worldwide to obtain new findings applicable to new biomedical implants and to support the biomedical industry. These attempts are being carried out in different R&D areas, such as: Materials, EBM Technology, Software. | Function | It must adapt to the patient's geometry, join, transfer load, provide support, must be porous to allow bone osteo-integration in the patient's body and bone growth, the manufacture in a single step of prostheses that combine solid and porous areas (scaffolds) | It relies on the previous experience of the design team (scientists, engineers, surgeons, etc.) The new custom implant design is commonly validated by Finite Element Analysis (FEA or FEM). Through 3D CAD programs, these scaffolds can be designed with the desired pore size, morphology, well-interconnected porosity, and gradual transition from solid (body implant) to porous (scaffold). | It relies on the previous experience of the design team (scientists, engineers, surgeons, etc.). Through 3D CAD programs, these scaffolds can be designed with the desired pore size, morphology, well-interconnected porosity, and gradual transition from solid (body implant) to porous (scaffold), as shown in Figure 24. Designers can control the implant design and have freedom in the design of scaffolds (multiple geometric solutions) for different pathologies. |
| GME6 | Implants | Bone implant | ME54, ME55 | illustrate the capabilities of AM in the design and manufacturing of medical implants. | Literature review | In the biomedical field, many efforts are being made to develop new materials and/or improve the properties of materials (metals and polymers) for biomedical applications. In addition, the evolution of manufacturing processes, coatings, and surface implant modification are notable areas of work. Nowadays, metals are the best choice for long-term load-bearing prostheses. For this reason, the development of new metallic biomaterials for long-term prostheses and the adaptation of manufacturing processes to achieve the best possible properties is a matter of great interest. * Improvement of biocompatibility and modulus. * A ray strategy called MultiBeam has been launched, which allows for the production of finer details and a better surface finish. * New 3D CAD tools will allow for more freedom in design. Most commonly used commercial 3D CAD tools are designed for manufacturing processes and do not easily allow for the design of new concepts with complex geometries, such as scaffolds, fractals, or bionic features. * Expert systems for process planning automation. * Specific CAE tools for scanner-to-part reconstruction, automatic guidance in implant design in STL format, assisted automated topological optimization of network structures, etc. | The technology EBM has proven to be a very powerful tool for the manufacturing of high value-added products. At the same time, EBM is a relatively recent manufacturing technology and has much more room for improvement. For both reasons, there are different R&D attempts being carried out worldwide to obtain new findings applicable to new biomedical implants and to support the biomedical industry. These attempts are being carried out in different R&D areas, such as: Materials, EBM Technology, Software. | Dimensions/ customization | It should be of the same dimensions and geometry as the patient. | the design process starts from the scanned patient information (Computed Axial Tomography (CAT), Magnetic Resonance Imaging (MRI) or X-ray). | Decreased stress protection effect. In the case of standard implants, the size chosen by the surgeon does not adapt perfectly to the patient's biomechanics, and there will be a higher probability of bone resorption since this standard implant does not adequately transfer loads to the nearby bone tissue. Avoid manual adjustment of the standard implant during surgery. When deformed, the implant has been damaged as the material has undergone plastic deformation. This deformation produces local strengthening while reducing ductility, leading to a change in implant behavior. This is especially critical in long-term prostheses that support cyclic loads, as this plastic deformation can reduce fatigue and the overall durability of the implant. The implant has undergone a very damaging deformation equivalent to many cycles under normal conditions of use. The second drawback is that corrosion may occur because plastic deformation can break the passive layer of the contact area (pitting corrosion). |
| GME6 | Implants | Bone implant | ME54, ME55 | illustrate the capabilities of AM in the design and manufacturing of medical implants. | Literature review | In the biomedical field, many efforts are being made to develop new materials and/or improve the properties of materials (metals and polymers) for biomedical applications. In addition, the evolution of manufacturing processes, coatings, and surface implant modification are notable areas of work. Nowadays, metals are the best choice for long-term load-bearing prostheses. For this reason, the development of new metallic biomaterials for long-term prostheses and the adaptation of manufacturing processes to achieve the best possible properties is a matter of great interest. * Improvement of biocompatibility and modulus. * A ray strategy called MultiBeam has been launched, which allows for the production of finer details and a better surface finish. * New 3D CAD tools will allow for more freedom in design. Most commonly used commercial 3D CAD tools are designed for manufacturing processes and do not easily allow for the design of new concepts with complex geometries, such as scaffolds, fractals, or bionic features. * Expert systems for process planning automation. * Specific CAE tools for scanner-to-part reconstruction, automatic guidance in implant design in STL format, assisted automated topological optimization of network structures, etc. | The technology EBM has proven to be a very powerful tool for the manufacturing of high value-added products. At the same time, EBM is a relatively recent manufacturing technology and has much more room for improvement. For both reasons, there are different R&D attempts being carried out worldwide to obtain new findings applicable to new biomedical implants and to support the biomedical industry. These attempts are being carried out in different R&D areas, such as: Materials, EBM Technology, Software. | Strength | It should be resistant, durable, withstand wear (joints), lightweight, and have similar rigidity to the original tissue (reduce rigidity). The implant should transfer the loads it supports to the bone to prevent its resorption. The manufacture in a single step of prostheses that combine solid and porous areas (scaffolds). | The new custom design of the implant is commonly validated by Finite Element Analysis (FEA or FEM). In the case of a structural analysis, the solution shows a distribution in a three-dimensional map with the level of stress (strain, displacement, etc.) along the geometry. | Custom implants could reduce the negative effect of protection against stress. Shielding against stress refers to the reduction of bone density (osteopenia) as a result of the elimination of normal bone stress by an implant (e.g., the femoral component of a hip prosthesis). According to Wolff's law (Wolff, 1986), the bone of a healthy person or animal remodels in response to the loads it is subjected to. Therefore, if the bone load decreases, the bone will become less dense and weaker because there is no stimulus for the continuous remodeling required to maintain bone mass. During the custom implant design process, shielding against stress can be taken into account and minimized through different designs and structural FEA analysis to achieve. |
| GME6 | Implants | Bone implant | ME54, ME55 | illustrate the capabilities of AM in the design and manufacturing of medical implants. | Literature review | In the biomedical field, many efforts are being made to develop new materials and/or improve the properties of materials (metals and polymers) for biomedical applications. In addition, the evolution of manufacturing processes, coatings, and surface implant modification are notable areas of work. Nowadays, metals are the best choice for long-term load-bearing prostheses. For this reason, the development of new metallic biomaterials for long-term prostheses and the adaptation of manufacturing processes to achieve the best possible properties is a matter of great interest. * Improvement of biocompatibility and modulus. * A ray strategy called MultiBeam has been launched, which allows for the production of finer details and a better surface finish. * New 3D CAD tools will allow for more freedom in design. Most commonly used commercial 3D CAD tools are designed for manufacturing processes and do not easily allow for the design of new concepts with complex geometries, such as scaffolds, fractals, or bionic features. * Expert systems for process planning automation. * Specific CAE tools for scanner-to-part reconstruction, automatic guidance in implant design in STL format, assisted automated topological optimization of network structures, etc. | The technology EBM has proven to be a very powerful tool for the manufacturing of high value-added products. At the same time, EBM is a relatively recent manufacturing technology and has much more room for improvement. For both reasons, there are different R&D attempts being carried out worldwide to obtain new findings applicable to new biomedical implants and to support the biomedical industry. These attempts are being carried out in different R&D areas, such as: Materials, EBM Technology, Software. | Material | It should be resistant, durable, withstand wear (joints), lightweight, and have a similar rigidity to the original fabric. The manufacture in a single step of prostheses that combine solid and porous areas (scaffolds). | Ti64, Ti64 ELI and Ti grade 2, CoCr ASTM F75 (Wear-resistant joints) | Custom implants could reduce the negative effect of stress shielding. Stress shielding refers to the reduction in bone density (osteopenia) as a result of the removal of normal bone stress by an implant (e.g., the femoral component of a hip prosthesis). According to Wolff's law (Wolff, 1986), the bone of a healthy person or animal remodels in response to the loads it is subjected to. Therefore, if the bone load decreases, the bone will become less dense and weaker because there is no stimulus for the continuous remodeling required to maintain bone mass. During the design process of the custom implant, stress shielding can be taken into account and minimized through different designs and structural FEM analysis to achieve. Wear resistance applies to joints such as knees, elbows, subjected to wear due to load variation and continuous use, and fatigue resistance applies to any support piece that will be subjected to dynamic loading. |
| GME6 | Implants | Bone implant | ME54, ME55 | illustrate the capabilities of AM in the design and manufacturing of medical implants. | Literature review | In the biomedical field, many efforts are being made to develop new materials and/or improve the properties of materials (metals and polymers) for biomedical applications. In addition, the evolution of manufacturing processes, coatings, and surface implant modification are notable areas of work. Nowadays, metals are the best choice for long-term load-bearing prostheses. For this reason, the development of new metallic biomaterials for long-term prostheses and the adaptation of manufacturing processes to achieve the best possible properties is a matter of great interest. * Improvement of biocompatibility and modulus. * A ray strategy called MultiBeam has been launched, which allows for the production of finer details and a better surface finish. * New 3D CAD tools will allow for more freedom in design. Most commonly used commercial 3D CAD tools are designed for manufacturing processes and do not easily allow for the design of new concepts with complex geometries, such as scaffolds, fractals, or bionic features. * Expert systems for process planning automation. * Specific CAE tools for scanner-to-part reconstruction, automatic guidance in implant design in STL format, assisted automated topological optimization of network structures, etc. | The technology EBM has proven to be a very powerful tool for the manufacturing of high value-added products. At the same time, EBM is a relatively recent manufacturing technology and has much more room for improvement. For both reasons, there are different R&D attempts being carried out worldwide to obtain new findings applicable to new biomedical implants and to support the biomedical industry. These attempts are being carried out in different R&D areas, such as: Materials, EBM Technology, Software. | Manufacturing and Assembly | The translated value of the provided data Las tolerancias de proceso y fabricación deben ser suficientes. in English is Process and manufacturing tolerances must be sufficient. | The EBM process is chosen. Like in every reverse engineering process, there is an error introduced during the reconstruction. In the scanning process, the maximum introduced deviation was 1.4 mm - most of the model points have a deviation between 0.45 and 0.65 mm (Figure 9, left). On the other hand, the manufacturing process reproduces the model with a deviation lower than 0.15 mm in over 80% of the points. | The materials it handles have resistance and durability, and tolerances, roughness have been tested in multiple case studies in animals and humans. |
| GME6 | Implants | Bone implant | ME54, ME55 | illustrate the capabilities of AM in the design and manufacturing of medical implants. | Literature review | In the biomedical field, many efforts are being made to develop new materials and/or improve the properties of materials (metals and polymers) for biomedical applications. In addition, the evolution of manufacturing processes, coatings, and surface implant modification are notable areas of work. Nowadays, metals are the best choice for long-term load-bearing prostheses. For this reason, the development of new metallic biomaterials for long-term prostheses and the adaptation of manufacturing processes to achieve the best possible properties is a matter of great interest. * Improvement of biocompatibility and modulus. * A ray strategy called MultiBeam has been launched, which allows for the production of finer details and a better surface finish. * New 3D CAD tools will allow for more freedom in design. Most commonly used commercial 3D CAD tools are designed for manufacturing processes and do not easily allow for the design of new concepts with complex geometries, such as scaffolds, fractals, or bionic features. * Expert systems for process planning automation. * Specific CAE tools for scanner-to-part reconstruction, automatic guidance in implant design in STL format, assisted automated topological optimization of network structures, etc. | The technology EBM has proven to be a very powerful tool for the manufacturing of high value-added products. At the same time, EBM is a relatively recent manufacturing technology and has much more room for improvement. For both reasons, there are different R&D attempts being carried out worldwide to obtain new findings applicable to new biomedical implants and to support the biomedical industry. These attempts are being carried out in different R&D areas, such as: Materials, EBM Technology, Software. | Costs and deadlines | Reduce manufacturing times, reduce surgical intervention times, and postoperative time. | Less than 10 hours in manufacturing per EBM for each board (case studies) | Less exposure to external bacteriological agents (even in a sterile atmosphere) leads to a lower probability of infection (one of the main risks during the traumatological and orthopedic phase). A lower dose of anesthesia is necessary. This factor can be critical in certain cases. Less recovery time in the hospital. Therefore, the costs of the surgical operation are reduced and more patients can be treated. |
| GME6 | Implants | Bone implant | ME54, ME55 | illustrate the capabilities of AM in the design and manufacturing of medical implants. | Literature review | In the biomedical field, many efforts are being made to develop new materials and/or improve the properties of materials (metals and polymers) for biomedical applications. In addition, the evolution of manufacturing processes, coatings, and surface implant modification are notable areas of work. Nowadays, metals are the best choice for long-term load-bearing prostheses. For this reason, the development of new metallic biomaterials for long-term prostheses and the adaptation of manufacturing processes to achieve the best possible properties is a matter of great interest. * Improvement of biocompatibility and modulus. * A ray strategy called MultiBeam has been launched, which allows for the production of finer details and a better surface finish. * New 3D CAD tools will allow for more freedom in design. Most commonly used commercial 3D CAD tools are designed for manufacturing processes and do not easily allow for the design of new concepts with complex geometries, such as scaffolds, fractals, or bionic features. * Expert systems for process planning automation. * Specific CAE tools for scanner-to-part reconstruction, automatic guidance in implant design in STL format, assisted automated topological optimization of network structures, etc. | The technology EBM has proven to be a very powerful tool for the manufacturing of high value-added products. At the same time, EBM is a relatively recent manufacturing technology and has much more room for improvement. For both reasons, there are different R&D attempts being carried out worldwide to obtain new findings applicable to new biomedical implants and to support the biomedical industry. These attempts are being carried out in different R&D areas, such as: Materials, EBM Technology, Software. | Security | It should be bio-compatible (ISO, 2010), (ASTM, 2010), ASTM F75 | Selected and testing of materials that comply with the corresponding standard for medical implants (ISO, 2010), (ASTM, 2010), ASTM F75 | The materials it handles have resistance and durability, bio-compatibility, and tolerances, roughness, bio-compatibility, has been tested in multiple case studies in animals and humans. |
| GME6 | Implants | Bone implant | ME54, ME55 | illustrate the capabilities of AM in the design and manufacturing of medical implants. | Literature review | In the biomedical field, many efforts are being made to develop new materials and/or improve the properties of materials (metals and polymers) for biomedical applications. In addition, the evolution of manufacturing processes, coatings, and surface implant modification are notable areas of work. Nowadays, metals are the best choice for long-term load-bearing prostheses. For this reason, the development of new metallic biomaterials for long-term prostheses and the adaptation of manufacturing processes to achieve the best possible properties is a matter of great interest. * Improvement of biocompatibility and modulus. * A ray strategy called MultiBeam has been launched, which allows for the production of finer details and a better surface finish. * New 3D CAD tools will allow for more freedom in design. Most commonly used commercial 3D CAD tools are designed for manufacturing processes and do not easily allow for the design of new concepts with complex geometries, such as scaffolds, fractals, or bionic features. * Expert systems for process planning automation. * Specific CAE tools for scanner-to-part reconstruction, automatic guidance in implant design in STL format, assisted automated topological optimization of network structures, etc. | The technology EBM has proven to be a very powerful tool for the manufacturing of high value-added products. At the same time, EBM is a relatively recent manufacturing technology and has much more room for improvement. For both reasons, there are different R&D attempts being carried out worldwide to obtain new findings applicable to new biomedical implants and to support the biomedical industry. These attempts are being carried out in different R&D areas, such as: Materials, EBM Technology, Software. | Legal Aspects | It should be bio-compatible (ISO, 2010), (ASTM, 2010), ASTM F75 | Selected and testing of materials that comply with the corresponding standard for medical implants (ISO, 2010), (ASTM, 2010), ASTM F75 | The materials it handles have resistance and durability, bio-compatibility, and tolerances, roughness, bio-compatibility, has been tested in multiple case studies in animals and humans. |
| GME6 | Implants | - | ME38 | Three-dimensional printing (3DP) is gaining increasing recognition as a technique that will transform the landscape of surgical practice. It allows for the rapid conversion of anatomical images into physical objects, which are being used across a variety of surgical specialties. It is not clear which groups are leading the way in finding novel ways to use the technology and what the technology is specifically being used for. The aim of this article was to review the current applications of 3DP in modern surgical practice. | A electronic search was conducted in MEDLINE, EMBASE and PsycINFO for terms related to 3DP. These were then examined for their relevance and the practical applications of the technology in surgery. | Results: Initially, 488 articles were found, and these were finally reduced to 93 full-text articles. It was determined that there were three main areas in which technology is being used for printing: (1) anatomical models, (2) surgical instruments, and (3) implants and prostheses. | The different specialties are at different stages in the use of technology. The costs of implementing technology and the time it takes for printing are important factors to consider before widespread use. In the foreseeable future, it is an exciting and interesting technology with the ability to radically change healthcare and revolutionize modern surgery. | - | - | - | - |
| GME6 | Implants | - | ME53 | In recent years, the use of three-dimensional printing (3DP) technology has gained momentum in spinal orthopedic surgery. Although research on this topic is still mainly limited to case reports and small cohort studies, it is evident that there are many avenues for 3DP innovation in the field. This review article aims to examine the current and emerging applications of 3DP in spinal surgery, as well as the challenges of 3DP production and the limitations of its use. | Literature review | The models of 3DP have been presented as useful tools for patient education, medical training, and preoperative planning. Intraoperatively, 3DP devices can serve as guides and surgical implants for the specific patient that improve surgical outcomes. However, the time, cost, and learning curve associated with building a 3DP model are significant barriers to its widespread use in spinal surgery. | Considering the costs and benefits of 3DP along with the various risks associated with different spinal procedures, 3DP technology is likely most valuable for complex or atypical cases of spinal disorders. Further research is justified to better understand how 3DP can and will impact spinal surgery. | - | - | - | - |
| GME6 | Implants | tissue implant | ME24 | We demonstrate how to manufacture the mold of soft prostheses with a low-cost desktop 3D printer. | The manufacturing method used is known as Scanning Printing Polishing Casting (SPPC). First, the anatomy is scanned with a 3D scanner (kinet for Windows), then a tissue casting mold is designed on the computer (Rhinoceros V4.0 software) and printed in ABS with a desktop 3D printer (FFF). Subsequently, a chemical polishing method is used (with acetone, the heater is turned on, the container containing the acetone is heated to a temperature higher than the boiling point of acetone (56.5°C), as it is set to 80°C for the experiment. The heated acetone vapor rises from the container and washes the ABS sample, the acetone will no longer evaporate when the pressure inside the sealed container reaches its saturated vapor pressure. A simple equation (Ideal Gas State Equation) was used to calculate the reasonable volume of acetone) to polish the casting mold, removing the stair-step effect and acquiring a smooth surface. Finally, the last step is to melt medical-grade silicone into the mold (Silicone part A and part B (Dongguan Hongfeng Silicone Materials Co., LTD) were mixed in a 1:1 ratio (weight:weight) and stirred for 2 minutes, and then the mixture was degassed in a vacuum chamber for 10-15 minutes). After the silicone is cured, the soft prostheses can be removed from the mold. Using the SPPC method, soft prostheses with a smooth surface and complicated structure can be manufactured at a low cost. | We proposed to manufacture soft prostheses with the help of a desktop 3D printer and achieved this goal. The printing costs of the negative mold were insignificant compared to traditional methods. This report demonstrates a cheap and convenient (SPPC) method of silicone prosthesis manufacturing. The stair-step effect caused by the desktop 3D printer is eliminated. | As an AI language model, I can help you with translation. Here is the translation of the provided data into English: As a result, the total cost of manufacturing the ear prosthesis is around S30, which is much lower than current methods of manufacturing soft prostheses. the translation may not be 100% accurate, as it is generated by an AI model. | - | - | - | - |
| GME6 | Implants | Bone implant | ME25 | The purpose of this work is to investigate the use of medical-grade polymethyl methacrylate (PMMA) in fused deposition modeling (FDM) for manufacturing customized freeform porous structures for various applications, including craniofacial reconstruction and orthopedic spacers. It also aims to examine the effects of different manufacturing conditions on the porosity and mechanical properties of PMMA samples. | The parameters for construction and procedures for properly and consistently extruding PMMA filament in FDM to build 3D structures were determined. Two experiments were conducted to examine the effects of different manufacturing conditions, including the cleaning frequency of the nozzles, layer orientation, and air gap (AG) (or distance between filament edges) on the mechanical properties and porosity of the fabricated structures. The samples were characterized using optical micrographs, and measurements of sample weight and dimensions were used to calculate porosity. The yield strength, deformation, and modulus of elasticity of the samples were determined through compression tests. | The results show that both the cleaning frequency of the tip (cleaning after every layer or cleaning after every ten layers) and the orientation of the layers (transverse or axial with respect to the applied compression load) used to manufacture the scaffolds have effects on the mechanical properties and resulting porosity. Samples manufactured in the transverse orientation with high frequency of cloth at the tip have higher compression strength and higher modulus than samples with low frequency of cloth at the tip (compression strength: 16 ^ 0.97 vs 13 ^ 0.71 MPa, modulus: 370 ^ 14 vs 313 ^ 29 MPa, for high vs low frequency of cloth at the tip, respectively). Additionally, samples manufactured in the transverse orientation have higher compression strength and modulus than those manufactured in the axial orientation (compression strength: 16 ^ 0.97 vs 13 ^ 0.83 MPa, modulus: 370 ^ 14 vs 281 ^ 22 MPa; for samples manufactured with one cloth per layer in the transverse and axial orientations, respectively). Overall, stiffness and yield strength decreased as porosity increased (compression strength: 12 ^ 0.71 to 7 ^ 0.95 MPa, modulus: 248 ^ 10 to 165 ^ 16 MPa, for samples with porosity ranging from 55 to 70 percent). As a demonstration, FDM is successfully used to manufacture patient-specific three-dimensional PMMA implants with variable densities, including the repair of cranial defects and femur models. | The necessary construction parameters for the successful manufacturing of FDM with medical grade PMMA filament (1/1600Ø) were developed using an FDM 3000. It was found that a liquefaction temperature and a wrap temperature of 235C and 55C, respectively, as well as a 60 percent increase in the model feed rate, were necessary to properly and consistently extrude the PMMA filament. Structures with different porosities and manufacturing conditions (tip scanning frequency and layer orientation) were produced, and their compression mechanical properties were examined. The results showed that both the tip cleaning frequency (cleaning every layer or every ten layers) and the layer orientation (transverse or axial with respect to the applied compression load) used to manufacture the structures, as well as the porosity (50-70 percent) of the structure, had an effect on the mechanical properties. Overall, the compression strength results of the porous PMMA structures manufactured (seven to 16 MPa, with a porosity of 50-70 percent) were in the range of trabecular bone (three to 15 MPa with a porosity of 70-90 percent). It is expected that as the porosity of the manufactured samples decreases, the compression strengths will approach those of molded PMMA bone cement (85-100 MPa), allowing for properties closer to cortical bone (130-180 MPa). Like other AM technologies, FDM allows for the ability to rapidly manufacture complex geometries with controlled architectures, and in the present case, the processing of PMMA in FDM will allow for the direct manufacturing of a variety of medical implants from medical imaging data. To demonstrate the success of manufacturing patient-specific PMMA 3D FDM models with variable densities, a model of a structure to repair a cranial defect and a model of a femur were manufactured. Implants for cranial repairs can be directly manufactured in FDM from medical imaging data and are designed with specific and controlled porosities. This methodology represents an alternative to the current use of PMMA in cranial reconstruction. The demonstrations showed the possibility of using FDM for the direct manufacturing of customized PMMA structures with variable and controlled porosities, and therefore adapted mechanical properties. Many additional opportunities will be enabled when this work is expanded to other biocompatible thermoplastic materials such as polypropylene, polycaprolactone, polyethylene, and other derived compounds that can be used to enhance mechanical properties and bioactivity. | Function | Use of PMMA filament for the design and manufacture of patient-specific implantable 3D models to repair cranial defects can be directly fabricated in PMMA from medical imaging data. The models can be manufactured with specific and controlled porosity, and this method represents a potentially beneficial alternative to the current use of PMMA in cranial reconstruction. Creation of temporary bone spacers or bone substitutes for infected open fractures and failed implants. | 3D CAD models can be generated from sets of medical image data acquired through CT or MRI scanners using image processing software such as Mimicsw (Materialise NV, Irvine, California). CAD models can then be adapted to create scaffolds with specific porosities using FDM technology. | The cranial models have porosities that can promote the transport of nutrients, waste, and biochemical signals (Blecha et al., 2009) and will prevent the accumulation of postoperative fluids and promote tissue growth (Kujala et al., 2003). These spacers preserve the tension of soft tissues and reduce dead space at the fracture site, and PMMA material can be combined with antibiotics to provide local antibiotic delivery and fight infection (Eufinger et al., 1995; Frutos Cabanillas et al., 2000; Pelletier et al., 2009). |
| GME6 | Implants | Bone implant | ME25 | The purpose of this work is to investigate the use of medical-grade polymethyl methacrylate (PMMA) in fused deposition modeling (FDM) for manufacturing customized freeform porous structures for various applications, including craniofacial reconstruction and orthopedic spacers. It also aims to examine the effects of different manufacturing conditions on the porosity and mechanical properties of PMMA samples. | The parameters for construction and procedures for properly and consistently extruding PMMA filament in FDM to build 3D structures were determined. Two experiments were conducted to examine the effects of different manufacturing conditions, including the cleaning frequency of the nozzles, layer orientation, and air gap (AG) (or distance between filament edges) on the mechanical properties and porosity of the fabricated structures. The samples were characterized using optical micrographs, and measurements of sample weight and dimensions were used to calculate porosity. The yield strength, deformation, and modulus of elasticity of the samples were determined through compression tests. | The results show that both the cleaning frequency of the tip (cleaning after every layer or cleaning after every ten layers) and the orientation of the layers (transverse or axial with respect to the applied compression load) used to manufacture the scaffolds have effects on the mechanical properties and resulting porosity. Samples manufactured in the transverse orientation with high frequency of cloth at the tip have higher compression strength and higher modulus than samples with low frequency of cloth at the tip (compression strength: 16 ^ 0.97 vs 13 ^ 0.71 MPa, modulus: 370 ^ 14 vs 313 ^ 29 MPa, for high vs low frequency of cloth at the tip, respectively). Additionally, samples manufactured in the transverse orientation have higher compression strength and modulus than those manufactured in the axial orientation (compression strength: 16 ^ 0.97 vs 13 ^ 0.83 MPa, modulus: 370 ^ 14 vs 281 ^ 22 MPa; for samples manufactured with one cloth per layer in the transverse and axial orientations, respectively). Overall, stiffness and yield strength decreased as porosity increased (compression strength: 12 ^ 0.71 to 7 ^ 0.95 MPa, modulus: 248 ^ 10 to 165 ^ 16 MPa, for samples with porosity ranging from 55 to 70 percent). As a demonstration, FDM is successfully used to manufacture patient-specific three-dimensional PMMA implants with variable densities, including the repair of cranial defects and femur models. | The necessary construction parameters for the successful manufacturing of FDM with medical grade PMMA filament (1/1600Ø) were developed using an FDM 3000. It was found that a liquefaction temperature and a wrap temperature of 235C and 55C, respectively, as well as a 60 percent increase in the model feed rate, were necessary to properly and consistently extrude the PMMA filament. Structures with different porosities and manufacturing conditions (tip scanning frequency and layer orientation) were produced, and their compression mechanical properties were examined. The results showed that both the tip cleaning frequency (cleaning every layer or every ten layers) and the layer orientation (transverse or axial with respect to the applied compression load) used to manufacture the structures, as well as the porosity (50-70 percent) of the structure, had an effect on the mechanical properties. Overall, the compression strength results of the porous PMMA structures manufactured (seven to 16 MPa, with a porosity of 50-70 percent) were in the range of trabecular bone (three to 15 MPa with a porosity of 70-90 percent). It is expected that as the porosity of the manufactured samples decreases, the compression strengths will approach those of molded PMMA bone cement (85-100 MPa), allowing for properties closer to cortical bone (130-180 MPa). Like other AM technologies, FDM allows for the ability to rapidly manufacture complex geometries with controlled architectures, and in the present case, the processing of PMMA in FDM will allow for the direct manufacturing of a variety of medical implants from medical image data. To demonstrate the success of manufacturing patient-specific PMMA 3D FDM models with variable densities, a model of a structure to repair a cranial defect and a model of a femur were manufactured. Implants for cranial repairs can be directly manufactured in FDM from medical image data and are designed with specific and controlled porosities. This methodology represents an alternative to the current use of PMMA in cranial reconstruction. The demonstrations showed the possibility of using FDM for the direct manufacturing of customized PMMA structures with variable and controlled porosities, and therefore adapted mechanical properties. Many additional opportunities will be enabled when this work is expanded to other biocompatible thermoplastic materials such as polypropylene, polycaprolactone, polyethylene, and other derived compounds that can be used to enhance mechanical properties and bioactivity. | Dimensions/Customization | The implant must replicate the size and shape of specific defects in the patient. | 3D CAD models can be generated from sets of medical image data acquired through CT or MRI scanners using image processing software such as Mimicsw (Materialise NV, Irvine, California). CAD models can then be adapted to create scaffolds with specific porosities using FDM technology. | Cranial models have porosities that can facilitate the transport of nutrients, waste, and biochemical signals (Blecha et al., 2009) and will prevent the accumulation of postoperative fluids and promote tissue growth (Kujala et al., 2003). |
| GME6 | Implants | Bone implant | ME25 | The purpose of this work is to investigate the use of medical-grade polymethyl methacrylate (PMMA) in fused deposition modeling (FDM) for manufacturing customized freeform porous structures for various applications, including craniofacial reconstruction and orthopedic spacers. It also aims to examine the effects of different manufacturing conditions on the porosity and mechanical properties of PMMA samples. | The parameters for construction and procedures for properly and consistently extruding PMMA filament in FDM to build 3D structures were determined. Two experiments were conducted to examine the effects of different manufacturing conditions, including the cleaning frequency of the nozzles, layer orientation, and air gap (AG) (or distance between filament edges) on the mechanical properties and porosity of the fabricated structures. The samples were characterized using optical micrographs, and measurements of sample weight and dimensions were used to calculate porosity. The yield strength, deformation, and modulus of elasticity of the samples were determined through compression tests. | The results show that both the cleaning frequency of the tip (cleaning after every layer or cleaning after every ten layers) and the orientation of the layers (transverse or axial with respect to the applied compression load) used to manufacture the scaffolds have effects on the mechanical properties and resulting porosity. Samples manufactured in the transverse orientation with high frequency of cloth at the tip have higher compression strength and higher modulus than samples with low frequency of cloth at the tip (compression strength: 16 ^ 0.97 vs 13 ^ 0.71 MPa, modulus: 370 ^ 14 vs 313 ^ 29 MPa, for high vs low frequency of cloth at the tip, respectively). Additionally, samples manufactured in the transverse orientation have higher compression strength and modulus than those manufactured in the axial orientation (compression strength: 16 ^ 0.97 vs 13 ^ 0.83 MPa, modulus: 370 ^ 14 vs 281 ^ 22 MPa; for samples manufactured with one cloth per layer in transverse and axial orientations, respectively). In general, stiffness and yield strength decreased as porosity increased (compression strength: 12 ^ 0.71 to 7 ^ 0.95 MPa, modulus: 248 ^ 10 to 165 ^ 16 MPa, for samples with porosity ranging from 55 to 70 percent). As a demonstration, FDM is successfully used to manufacture patient-specific three-dimensional PMMA implants with variable densities, including the repair of cranial defects and femur models. | The necessary construction parameters for the successful manufacturing of FDM with medical grade PMMA filament (1/1600Ø) were developed using an FDM 3000. It was found that a liquefaction temperature and a wrap temperature of 235C and 55C, respectively, as well as a 60 percent increase in the model feed rate, were necessary to properly and consistently extrude the PMMA filament. Structures with different porosities and manufacturing conditions (tip scanning frequency and layer orientation) were produced, and their compression mechanical properties were examined. The results showed that both the tip cleaning frequency (cleaning every layer or every ten layers) and the layer orientation (transverse or axial with respect to the applied compression load) used to manufacture the structures, as well as the porosity (50-70 percent) of the structure, had an effect on the mechanical properties. Overall, the compression strength results of the porous PMMA structures manufactured (seven to 16 MPa, with a porosity of 50-70 percent) were in the range of trabecular bone (three to 15 MPa with a porosity of 70-90 percent). It is expected that as the porosity of the manufactured samples decreases, the compression strengths will approach those of molded PMMA bone cement (85-100 MPa), allowing for properties closer to cortical bone (130-180 MPa). Like other AM technologies, FDM allows for the ability to rapidly manufacture complex geometries with controlled architectures, and in the present case, the processing of PMMA in FDM will allow for the direct manufacturing of a variety of medical implants from medical imaging data. To demonstrate the success of manufacturing patient-specific PMMA 3D FDM models with variable densities, a model of a structure to repair a cranial defect and a model of a femur were manufactured. Implants for cranial repairs can be directly manufactured in FDM from medical imaging data and are designed with specific and controlled porosities. This methodology represents an alternative to the current use of PMMA in cranial reconstruction. The demonstrations showed the possibility of using FDM for the direct manufacturing of customized PMMA structures with variable and controlled porosities, and therefore adapted mechanical properties. Many additional opportunities will be enabled when this work is expanded to other biocompatible thermoplastic materials such as polypropylene, polycaprolactone, polyethylene, and other derived compounds that can be used to enhance mechanical properties and bioactivity. | Strength | The material and manufacturing parameters must be such that they allow the production of implants with resistances similar to those of the bone tissues they are going to replace. | ||
| GME6 | Implants | Bone implant | ME25 | The purpose of this work is to investigate the use of medical-grade polymethyl methacrylate (PMMA) in fused deposition modeling (FDM) for manufacturing customized freeform porous structures for various applications, including craniofacial reconstruction and orthopedic spacers. It also aims to examine the effects of different manufacturing conditions on the porosity and mechanical properties of PMMA samples. | The parameters for construction and procedures for properly and consistently extruding PMMA filament in FDM to build 3D structures were determined. Two experiments were conducted to examine the effects of different manufacturing conditions, including the cleaning frequency of the nozzles, layer orientation, and air gap (AG) (or distance between filament edges) on the mechanical properties and porosity of the fabricated structures. The samples were characterized using optical micrographs, and measurements of sample weight and dimensions were used to calculate porosity. The yield strength, deformation, and modulus of elasticity of the samples were determined through compression tests. | The results show that both the cleaning frequency of the tip (cleaning after every layer or cleaning after every ten layers) and the orientation of the layers (transverse or axial with respect to the applied compression load) used to manufacture the scaffolds have effects on the mechanical properties and resulting porosity. Samples manufactured in the transverse orientation with high cleaning frequency at the tip have higher compression strength and a higher modulus than samples with low cleaning frequency at the tip (compression strength: 16 +/- 0.97 vs 13 +/- 0.71 MPa, modulus: 370 +/- 14 vs 313 +/- 29 MPa, for high vs low cleaning frequency at the tip, respectively). Additionally, samples manufactured in the transverse orientation have higher compression strength and modulus than those manufactured in the axial orientation (compression strength: 16 +/- 0.97 vs 13 +/- 0.83 MPa, modulus: 370 +/- 14 vs 281 +/- 22 MPa; for samples manufactured with tip cleaning per layer in the transverse and axial orientations, respectively). Overall, stiffness and elastic limit decreased as porosity increased (compression strength: 12 +/- 0.71 to 7 +/- 0.95 MPa, modulus: 248 +/- 10 to 165 +/- 16 MPa, for samples with porosity ranging from 55 to 70 percent). As a demonstration, FDM is successfully used to manufacture patient-specific three-dimensional PMMA implants with variable densities, including the repair of cranial defects and femur models. | The necessary construction parameters for the successful manufacturing of FDM with medical grade PMMA filament (1/1600Ø) were developed using an FDM 3000. It was found that a liquefaction temperature and a wrap temperature of 235C and 55C, respectively, as well as a 60 percent increase in the model feed rate, were necessary to properly and consistently extrude the PMMA filament. Structures with different porosities and manufacturing conditions (tip scanning frequency and layer orientation) were produced, and their compression mechanical properties were examined. The results showed that both the tip cleaning frequency (cleaning every layer or every ten layers) and the layer orientation (transverse or axial with respect to the applied compression load) used to manufacture the structures, as well as the porosity (50-70 percent) of the structure, had an effect on the mechanical properties. Overall, the compression strength results of the porous PMMA structures manufactured (seven to 16 MPa, with a porosity of 50-70 percent) were in the range of trabecular bone (three to 15 MPa with a porosity of 70-90 percent). It is expected that as the porosity of the manufactured samples decreases, the compression strengths will approach those of molded PMMA bone cement (85-100 MPa), allowing for properties closer to cortical bone (130-180 MPa). Like other AM technologies, FDM allows for the ability to rapidly manufacture complex geometries with controlled architectures, and in the present case, the processing of PMMA in FDM will allow for the direct manufacturing of a variety of medical implants from medical image data. To demonstrate the success of manufacturing patient-specific PMMA 3D FDM models with variable densities, a model of a structure to repair a cranial defect and a model of a femur were manufactured. Implants for cranial repairs can be directly manufactured in FDM from medical image data and are designed with specific and controlled porosities. This methodology represents an alternative to the current use of PMMA in cranial reconstruction. The demonstrations showed the possibility of using FDM for the direct manufacturing of customized PMMA structures with variable and controlled porosities, and therefore adapted mechanical properties. Many additional opportunities will be enabled when this work is expanded to other biocompatible thermoplastic materials such as polypropylene, polycaprolactone, polyethylene, and other derived compounds that can be used to enhance mechanical properties and bioactivity. | Material | The material and manufacturing parameters must be such that they allow the production of implants with resistances and stiffness similar to that of the bone tissues they are going to replace. | Two experimental designs were carried out to determine the effect of different factors (manufacturing conditions and scaffold characteristics) on the mechanical properties of the fabricated structures. Experiment I analyzed the effect of tip rubbing frequency and construction orientation on the mechanical properties of the fabricated structures, while Experiment II evaluated the mechanical properties of samples with different porosity percentages. Each experimental condition consisted of six samples of square prisms with design dimensions of 7 x 7 x 13.5 mm. The scaffold structures were designed with a raster width of 0.406 mm, an SH of 0.254 mm, and a raster angle of 0°/90°. Air gap was varied to achieve different ranges of porosity. | Compression tests were performed on an Instron 5866 system (Instronw, Norwood, Massachusetts) following the guidelines provided in Zein et al. (2002) based on ASTM F451-08 standard. A 10 kN load cell was used, and a crosshead deformation rate of 1 mm/min was applied. Compression stress calculations were performed by dividing the applied force by the cross-sectional area of the original specimen. Strain was defined as the ratio of axial deformation to the height of the original sample. Compression stress-strain graphs were created from which various mechanical properties were obtained. Compressive yield strength was defined as the stress after which the initial linear region deviated from linearity; yield strain was defined as the stress associated with the compressive yield strength. Stiffness was defined as the elastic modulus, E, and was calculated from data representing the slope of the initial linear region. For all experiments, student t-tests were performed to compare the mean values of independent samples with a significance level of 0.05. Additionally, an ANOVA was conducted for the specimens used in Experiment II to compare the mean values of RW from six independent samples with a significance level of 0.05. The data shown in the graphs represent the means ± standard deviation. P values less than 0.05 were considered significant. The specimens for Experiment II were observed and imaged using a stereomicroscope (Leica MZ16, Leica Microsystems, Inc., Bannockburn, Illinois) equipped with a CCD camera (Retiga 2000R Fast 1394, QImaging Corp., Canada). Measurements for RW, AG, and SH were taken from digital images. Multiple images were captured to obtain at least five measurements per dimension per sample. |
| GME6 | Implants | Bone implant | ME25 | The purpose of this work is to investigate the use of medical-grade polymethyl methacrylate (PMMA) in fused deposition modeling (FDM) for manufacturing customized freeform porous structures for various applications, including craniofacial reconstruction and orthopedic spacers. It also aims to examine the effects of different manufacturing conditions on the porosity and mechanical properties of PMMA samples. | The parameters for construction and procedures for properly and consistently extruding PMMA filament in FDM to build 3D structures were determined. Two experiments were conducted to examine the effects of different manufacturing conditions, including the cleaning frequency of the nozzles, layer orientation, and air gap (AG) (or distance between filament edges) on the mechanical properties and porosity of the fabricated structures. The samples were characterized using optical micrographs, and measurements of sample weight and dimensions were used to calculate porosity. The yield strength, deformation, and modulus of elasticity of the samples were determined through compression tests. | The results show that both the cleaning frequency of the tip (cleaning after every layer or cleaning after every ten layers) and the orientation of the layers (transverse or axial with respect to the applied compression load) used to manufacture the scaffolds have effects on the mechanical properties and resulting porosity. Samples manufactured in the transverse orientation with high frequency of cloth at the tip have higher compression strength and higher modulus than samples with low frequency of cloth at the tip (compression strength: 16 ^ 0.97 vs 13 ^ 0.71 MPa, modulus: 370 ^ 14 vs 313 ^ 29 MPa, for high vs low frequency of cloth at the tip, respectively). Additionally, samples manufactured in the transverse orientation have higher compression strength and modulus than those manufactured in the axial orientation (compression strength: 16 ^ 0.97 vs 13 ^ 0.83 MPa, modulus: 370 ^ 14 vs 281 ^ 22 MPa; for samples manufactured with one cloth per layer in transverse and axial orientations, respectively). In general, stiffness and yield strength decreased as porosity increased (compression strength: 12 ^ 0.71 to 7 ^ 0.95 MPa, modulus: 248 ^ 10 to 165 ^ 16 MPa, for samples with porosity ranging from 55 to 70 percent). As a demonstration, FDM is successfully used to manufacture patient-specific three-dimensional PMMA implants with variable densities, including the repair of cranial defects and femur models. | The necessary construction parameters for the successful manufacturing of FDM with medical grade PMMA filament (1/1600Ø) were developed using an FDM 3000. It was found that a liquefaction temperature and a wrap temperature of 235C and 55C, respectively, as well as a 60 percent increase in the model feed rate, were necessary to properly and consistently extrude the PMMA filament. Structures with different porosities and manufacturing conditions (tip scanning frequency and layer orientation) were produced, and their compression mechanical properties were examined. The results showed that both the tip cleaning frequency (cleaning every layer or every ten layers) and the layer orientation (transverse or axial with respect to the applied compression load) used to manufacture the structures, as well as the porosity (50-70 percent) of the structure, had an effect on the mechanical properties. Overall, the compression strength results of the porous PMMA structures manufactured (seven to 16 MPa, with a porosity of 50-70 percent) were in the range of trabecular bone (three to 15 MPa with a porosity of 70-90 percent). It is expected that as the porosity of the manufactured samples decreases, the compression strengths will approach those of molded PMMA bone cement (85-100 MPa), allowing for properties closer to cortical bone (130-180 MPa). Like other AM technologies, FDM allows for the ability to rapidly manufacture complex geometries with controlled architectures, and in the present case, the processing of PMMA in FDM will allow for the direct manufacturing of a variety of medical implants from medical imaging data. To demonstrate the success of manufacturing patient-specific PMMA 3D FDM models with variable densities, a model of a structure to repair a cranial defect and a model of a femur were manufactured. Implants for cranial repairs can be directly manufactured in FDM from medical imaging data and are designed with specific and controlled porosities. This methodology represents an alternative to the current use of PMMA in cranial reconstruction. The demonstrations showed the possibility of using FDM for the direct manufacturing of customized PMMA structures with variable and controlled porosities, and therefore adapted mechanical properties. Many additional opportunities will be enabled when this work is expanded to other biocompatible thermoplastic materials such as polypropylene, polycaprolactone, polyethylene, and other derived compounds that can be used to enhance mechanical properties and bioactivity. | Manufacturing and Assembly | The process parameters must allow a ripple-free or deformation-free appearance that distorts the original shape of the CAD model, in the same way, they must be sufficient to achieve successful manufacturing. | The adaptation of the FDM 3000 system (Stratasys, Inc., Eden Prairie, Minnesota) to properly and consistently extrude PMMA filament (Biogeneral, Inc., San Diego, California) required identifying the parameters that allowed extrusion regardless of the material and determining the values of the appropriate parameters for PMMA. It was found that a liquefaction temperature of 235°C had the best mesh surfaces and minimal evidence of material residue for this wrapping temperature. Various wrapping temperatures were tested and it was found that 55°C was the best for minimizing material residue and improving mesh surfaces. Material feed rates were determined by running the default calibration method provided by Insight V3.5 cutting software (Stratasys, Inc.) until the appropriate raster widths corresponding to the diameter of the extrusion tips were achieved. | Through a trial and error process, different parameters of bed temperature, extruder temperature, and feed rate were tested: FDM extrusion mainly depends on the temperature of the liquefier, the temperature of the enclosure, and the feed rate of the material. Simple square prisms with T16 tips were fabricated, with an enclosure temperature of 75°C and various liquefaction temperatures, while closely monitoring the construction process. Relatively low temperatures, 180-210°C, failed to properly extrude PMMA as the filament would audibly break before entering the liquefiers. A temperature of 225°C allowed for the extrusion of PMMA but created extremely rigid rasters that did not adhere properly to the support material (ABS P400 R), causing the models to detach before being properly completed. Higher temperatures around 270°C created grids with visible wavy surfaces and caused excessive material residues that affected the geometry. Additionally, an excess of material flowed from the extrusion tips while the FDM system was not being constructed. |
| GME6 | Implants | Bone implant | ME25 | The purpose of this work is to investigate the use of medical-grade polymethyl methacrylate (PMMA) in fused deposition modeling (FDM) for manufacturing customized freeform porous structures for various applications, including craniofacial reconstruction and orthopedic spacers. It also aims to examine the effects of different manufacturing conditions on the porosity and mechanical properties of PMMA samples. | The parameters for construction and procedures for properly and consistently extruding PMMA filament in FDM to build 3D structures were determined. Two experiments were conducted to examine the effects of different manufacturing conditions, including the cleaning frequency of the nozzles, layer orientation, and air gap (AG) (or distance between filament edges) on the mechanical properties and porosity of the fabricated structures. The samples were characterized using optical micrographs, and measurements of sample weight and dimensions were used to calculate porosity. The yield strength, deformation, and modulus of elasticity of the samples were determined through compression tests. | The results show that both the cleaning frequency of the tip (cleaning after every layer or cleaning after every ten layers) and the orientation of the layers (transverse or axial with respect to the applied compression load) used to manufacture the scaffolds have effects on the mechanical properties and resulting porosity. Samples manufactured in the transverse orientation with high frequency of cloth at the tip have higher compression strength and higher modulus than samples with low frequency of cloth at the tip (compression strength: 16 ^ 0.97 vs 13 ^ 0.71 MPa, modulus: 370 ^ 14 vs 313 ^ 29 MPa, for high vs low frequency of cloth at the tip, respectively). Additionally, samples manufactured in the transverse orientation have higher compression strength and modulus than those manufactured in the axial orientation (compression strength: 16 ^ 0.97 vs 13 ^ 0.83 MPa, modulus: 370 ^ 14 vs 281 ^ 22 MPa; for samples manufactured with one cloth per layer in transverse and axial orientations, respectively). In general, stiffness and yield strength decreased as porosity increased (compression strength: 12 ^ 0.71 to 7 ^ 0.95 MPa, modulus: 248 ^ 10 to 165 ^ 16 MPa, for samples with porosity ranging from 55 to 70 percent). As a demonstration, FDM is successfully used to manufacture patient-specific three-dimensional PMMA implants with variable densities, including the repair of cranial defects and femur models. | The necessary construction parameters for the successful manufacturing of FDM with medical grade PMMA filament (1/1600Ø) were developed using an FDM 3000. It was found that a liquefaction temperature and a wrap temperature of 235C and 55C, respectively, as well as a 60 percent increase in the model feed rate, were necessary to properly and consistently extrude the PMMA filament. Structures with different porosities and manufacturing conditions (tip scanning frequency and layer orientation) were produced, and their compression mechanical properties were examined. The results showed that both the tip cleaning frequency (cleaning every layer or every ten layers) and the layer orientation (transverse or axial with respect to the applied compression load) used to manufacture the structures, as well as the porosity (50-70 percent) of the structure, had an effect on the mechanical properties. Overall, the compression strength results of the porous PMMA structures manufactured (seven to 16 MPa, with a porosity of 50-70 percent) were in the range of trabecular bone (three to 15 MPa with a porosity of 70-90 percent). It is expected that as the porosity of the manufactured samples decreases, the compression strengths will approach those of molded PMMA bone cement (85-100 MPa), allowing for properties closer to cortical bone (130-180 MPa). Like other AM technologies, FDM allows for the ability to rapidly manufacture complex geometries with controlled architectures, and in the present case, the processing of PMMA in FDM will allow for the direct manufacturing of a variety of medical implants from medical image data. To demonstrate the success of manufacturing patient-specific PMMA 3D FDM models with variable densities, a model of a structure to repair a cranial defect and a model of a femur were manufactured. Implants for cranial repairs can be directly manufactured in FDM from medical image data and are designed with specific and controlled porosities. This methodology represents an alternative to the current use of PMMA in cranial reconstruction. The demonstrations showed the possibility of using FDM for the direct manufacturing of customized PMMA structures with variable and controlled porosities, and therefore adapted mechanical properties. Many additional opportunities will be enabled when this work is expanded to other biocompatible thermoplastic materials such as polypropylene, polycaprolactone, polyethylene, and other derived compounds that can be used to enhance mechanical properties and bioactivity. | Security | The material must be bio-compatible. | PMMA filament is used and manufacturing parameters are refined to make manufacturing feasible. | PMMA material is commonly used in bone implant applications that do not support loads (FDA-approved material) |
| GME6 | Implants | Bone implant | ME25 | The purpose of this work is to investigate the use of medical-grade polymethyl methacrylate (PMMA) in fused deposition modeling (FDM) for manufacturing customized freeform porous structures for various applications, including craniofacial reconstruction and orthopedic spacers. It also aims to examine the effects of different manufacturing conditions on the porosity and mechanical properties of PMMA samples. | The parameters for construction and procedures for properly and consistently extruding PMMA filament in FDM to build 3D structures were determined. Two experiments were conducted to examine the effects of different manufacturing conditions, including the cleaning frequency of the nozzles, layer orientation, and air gap (AG) (or distance between filament edges) on the mechanical properties and porosity of the fabricated structures. The samples were characterized using optical micrographs, and measurements of sample weight and dimensions were used to calculate porosity. The yield strength, deformation, and modulus of elasticity of the samples were determined through compression tests. | The results show that both the cleaning frequency of the tip (cleaning after every layer or cleaning after every ten layers) and the orientation of the layers (transverse or axial with respect to the applied compression load) used to manufacture the scaffolds have effects on the mechanical properties and resulting porosity. Samples manufactured in the transverse orientation with high frequency of cloth at the tip have higher compression strength and higher modulus than samples with low frequency of cloth at the tip (compression strength: 16 ^ 0.97 vs 13 ^ 0.71 MPa, modulus: 370 ^ 14 vs 313 ^ 29 MPa, for high vs low frequency of cloth at the tip, respectively). Additionally, samples manufactured in the transverse orientation have higher compression strength and modulus than those manufactured in the axial orientation (compression strength: 16 ^ 0.97 vs 13 ^ 0.83 MPa, modulus: 370 ^ 14 vs 281 ^ 22 MPa; for samples manufactured with one cloth per layer in transverse and axial orientations, respectively). In general, stiffness and yield strength decreased as porosity increased (compression strength: 12 ^ 0.71 to 7 ^ 0.95 MPa, modulus: 248 ^ 10 to 165 ^ 16 MPa, for samples with porosity ranging from 55 to 70 percent). As a demonstration, FDM is successfully used to manufacture patient-specific three-dimensional PMMA implants with variable densities, including the repair of cranial defects and femur models. | The necessary construction parameters for the successful manufacturing of FDM with medical grade PMMA filament (1/1600Ø) were developed using an FDM 3000. It was found that a liquefaction temperature and a wrap temperature of 235C and 55C, respectively, as well as a 60 percent increase in the model feed rate, were necessary to properly and consistently extrude the PMMA filament. Structures with different porosities and manufacturing conditions (tip scanning frequency and layer orientation) were produced, and their compression mechanical properties were examined. The results showed that both the tip cleaning frequency (cleaning every layer or every ten layers) and the layer orientation (transverse or axial with respect to the applied compression load) used to manufacture the structures, as well as the porosity (50-70 percent) of the structure, had an effect on the mechanical properties. Overall, the compression strength results of the porous PMMA structures manufactured (seven to 16 MPa, with a porosity of 50-70 percent) were in the range of trabecular bone (three to 15 MPa with a porosity of 70-90 percent). It is expected that as the porosity of the manufactured samples decreases, the compression strengths will approach those of molded PMMA bone cement (85-100 MPa), allowing for properties closer to cortical bone (130-180 MPa). Like other AM technologies, FDM allows for the ability to rapidly manufacture complex geometries with controlled architectures, and in the present case, the processing of PMMA in FDM will allow for the direct manufacturing of a variety of medical implants from medical image data. To demonstrate the success of manufacturing patient-specific PMMA 3D FDM models with variable densities, a model of a structure to repair a cranial defect and a model of a femur were manufactured. Implants for cranial repairs can be directly manufactured in FDM from medical image data and are designed with specific and controlled porosities. This methodology represents an alternative to the current use of PMMA in cranial reconstruction. The demonstrations showed the possibility of using FDM for the direct manufacturing of customized PMMA structures with variable and controlled porosities, and therefore adapted mechanical properties. Many additional opportunities will be enabled when this work is expanded to other biocompatible thermoplastic materials such as polypropylene, polycaprolactone, polyethylene, and other derived compounds that can be used to enhance mechanical properties and bioactivity. | Legal Aspects | The material must be bio-compatible. | PMMA filament is used and manufacturing parameters are refined to make manufacturing feasible. Many additional opportunities will be enabled when this work is expanded to other biocompatible thermoplastic materials such as polypropylene, polycaprolactone, polyethylene, and other derived compounds that can be used to enhance mechanical properties and bioactivity. | PMMA material is commonly used in bone implant applications that do not support loads (FDA approved material). |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method to produce molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Function | Molding bone defect in PMMA resin. | Design and manufacture Mold in PC, based on computed tomography data of the skull and bone defect of the patient. Molded implant in PMMA. | |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method for producing molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Dimensions | The mold must have the shape of the patient's defect. | Data: translate computed tomography (CT) data into a 3D model of the lesion to determine the geometry and dimensions of the required implant using CAD software | |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method to produce molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Customization | The mold must have the shape of the patient's defect. | Data: translate computed tomography (CT) data into a 3D model of the lesion to determine the geometry and dimensions of the required implant using CAD software | They were downloaded into medical image visualization software for editing InVesalius, (which is open-source software for the manipulation and visualization of medical images, reconstructing DICOM images into a 3D model, generating an STL file). The section of the skull damage was selected using CAD software (PowerSHAPE, Delcam, UK) that allows for the manipulation of triangular meshes. From the definition of the contour of the lesion, the patient's head wireframe was drawn and used as a reference to establish the curvature of the implant, which became its external surface. Then, the thickness of the implant was established using the conical surface procedure (Min and Dean, 2003). This gradual decrease method comprises, first, the generation of a surface; second, an internal surface is added, which is smaller and parallel to the external one. Finally, both surfaces are merged, creating a third one that will be in contact with the skull. Each design parameter was determined by the specific anatomy of the patient. In addition, the implant CAD was evaluated to ensure its assembly to the patients' anatomy. For this purpose, both the implant and the lesion were manufactured in polycarbonate using the FDM 400 mc Stratasys machine. The next step in this manufacturing process was the design of the mold that would replicate the implant's geometry. To simplify the mold design process, a model lesion was studied and its corresponding mold was designed in CAD. A two-piece core and cavity mold was created by extruding the CAD model of the implant from a solid rectangular prism, resulting in a hollowed block that adopts the shape of the implant. The parting line was defined, considering that the central part of the mold contains 100% of the required thickness of the implant to apply pressure to the mold cavity. This consideration was made to avoid trapped air and therefore the appearance of porosity in the implant. At this stage of the process, the 3D-CAD data of the mold is ready to be manufactured. |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method to produce molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Load | The mold must withstand the loads during molding. | A static analysis of the mold assembly was carried out to determine that the applied pressure would not break or deform the polycarbonate mold. Displacement and stress analysis were performed using finite element analysis software (SolidWorks, Dassault Systèmes SolidWorks Corporation, USA), applying a pressure of 220 KPa on a polycarbonate assembly of 100-100 mm [Figure 6(b)], which represents the size of the mold for this case study. The tensile strength and tensile modulus of the polycarbonate reported by the manufacturer are 68 and 2,300 MPa, respectively. The objective of this analysis was to determine the minimum thickness of the mold required to achieve a maximum deformation of 80 m at its center, which was 12.5 mm. | A static analysis of the mold assembly was carried out to determine that the applied pressure would not break or deform the polycarbonate mold. Displacement and stress analysis were performed using finite element analysis software (SolidWorks, Dassault Systèmes SolidWorks Corporation, USA), applying a pressure of 220 KPa on a polycarbonate assembly of 100-100 mm [Figure 6(b)], which represents the size of the mold for this case study. The tensile strength and tensile modulus of the polycarbonate reported by the manufacturer are 68 and 2,300 MPa, respectively. The objective of this analysis was to determine the minimum thickness of the mold required to achieve a maximum deformation of 80 m at its center, which was 12.5 mm. |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method to produce molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Material | The mold must withstand the molding loads and the temperature of the PMMA resin, the PC must be biocompatible, and the demolding of the PMMA must also be facilitated. | PC manufactured in a Fortus, 400mc of Stratasys (FDM). The PMMA used for the manufacture of the implants was a commercial radiotransparent two-component bone cement (NicTon, Mexico). Polyvinyl alcohol (PVA) was used as a separation medium. | The material selection for the FDM-manufactured mold was based on the maximum temperature that the PMMA polymerization reaction can reach. Vallo (2000) reported a maximum temperature of 131.79°C for commercial PMMA bone cement with a cement thickness of 8 mm; in this study, the average implant thickness was 7.4 mm. Polycarbonate was the chosen model material within the available Stratasys Fortus 400 mc, as it has a heat deflection temperature of 138°C and is also chemically resistant to methyl methacrylate monomer. The mold was fabricated using breakaway support material (PCS) and a layer height of 0.254 mm (T16). The powder component consisted of PMMA powder, initiator (benzoyl peroxide), and other elements such as plasticizers, opacifiers, and pigments. Consequently, the liquid component was methyl methacrylate monomer, which may also include crosslinkers and stabilizers (Lee et al., 2002). The volume ratio used in the PMMA casting formulation was 2:1 powder/liquid. Polyvinyl alcohol (PVA) was used as a release medium. Each mold part was immersed in PVA for 1 minute, and the film was air-dried at room temperature (25°C) for 45 minutes. This procedure was repeated five times to achieve a homogeneous film with a thickness of 0.17 ± 0.02 mm, which was measured using a digital thickness gauge (model 700-118, Mitutoyo, Japan). Additionally, 15 ml of PMMA reaction mixture was prepared at room temperature and hand-mixed for 30 seconds. The reactive mixture was poured into the molds and closed with a 'C'-shaped clamp; two metal plates were placed between the clamp and the mold to evenly distribute the applied force. The molds containing the PMMA mixture were placed in an oven at 90°C for 3 hours. Once the curing time was completed, the PMMA pieces were manually removed from the mold and polished. |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method for producing molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Manufacturing and Assembly | The implant should not have porosity or roughness according to standard 12 of the American Dental Association (ADA) | PC manufactured on a Stratasys Fortus 400mc (FDM). After polishing the implant, the porosity of the implant was visually tested according to the American Dental Association (ADA) standard 12; there was no porosity in the manufactured implant. | For the clinical case study, the PMMA implant obtained using a mold manufactured with FDM showed good transparency and porosity visually evaluated; in other words, no air bubbles were detected inside the implant. |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method for producing molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | assembly | The implant must be adjusted to fit the patient's defect, during molding there must be evacuation of liquid resin and avoid the formation of air bubbles. | The implant was designed using the conical surface method. The CAD design of the implant was evaluated to ensure its assembly to the patients' anatomy. Care was taken to ensure that the mold cap defined the implant's geometry through a relief, so that it pushed out any excess reactive material and air when packed; thus, there would be no bubbles or air voids in the implant. | Printed models of implant and skull are tested, both their assembly and fit. A torque wrench (Cedar DID-4 Lightweight Digital Torque Tester, Sugisaki Meter Co., Ltd, Japan) was used to advance the clamp screw in C until the axial force applied to the metal plates was equivalent to 220 KPa. The mold cap defined the implant's geometry by means of a relief, so that it pushed all the excess reactive material and air when packed; thus, there would be no bubbles or air voids in the implant. showed a better fit than that produced by the conventional process. |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method for producing molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Costs and deadlines | Reduce manufacturing times compared to conventional methods. | The whole process usually takes between 5 and 10 weeks, including delays in third-party services and the time needed for the patient to obtain financial resources. The integration of this manufacturing process into the production of PMMA implants for craniofacial reconstruction reduced the production time of PMMA implants by decreasing the number of steps from six to four. The production time of the implant for the case study was five weeks by the traditional method and 15 hours by the mold-based manufacturing process produced with AM. This was achieved by: eliminating the manufacturing of the model lesion; eliminating the wax pattern and hand-producing stone molds; and adjusting the polymerization process according to the new properties of the mold material. All these improvements resulted in a 45.5% reduction in time compared to the process currently used by HGM. | This was achieved by: eliminating the manufacturing of the model's injury; eliminating the wax pattern and producing stone molds by hand; adjusting the polymerization process according to the new properties of the mold material. |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method for producing molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Security | Materials must be biocompatible | PMMA was used for the implant and PC for the mold. For this study, the Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects, was followed. All patients were informed that their injuries would be used in this research and written informed consent was obtained. A 22-year-old male with a parietal-temporal lesion of 95 cm2 is the reported clinical case study. | |
| GME6 | Implants | Bone implant | 366 | It is suggested to use computer-aided design (CAD) technology and additive manufacturing (AM) for the production of custom molds, designed for the manufacturing of polymethyl methacrylate (PMMA) implants for craniofacial reconstruction in order to reduce manufacturing time. Although there are custom-made cranial prostheses available in the market with high technological level and cutting-edge materials, they are not always accessible to the general population in developing countries. | Computed tomography data was used to create a three-dimensional (3D) model of the patient's lesion by reconstructing Digital Imaging and Communications in Medicine (DICOM) images into a Standard Tessellation Language (STL) file, which was then used to design the corresponding implant using CAD software. Consequently, a two-piece core and cavity molds were also designed to replicate the implant's geometry in CAD. The 3D-CAD data was sent to an Additive Manufacturing (AM) machine, and the molds were fabricated using polycarbonate as a thermoplastic material. A reactive mixture was poured into the molds to produce PMMA directly, and it was allowed to polymerize until cured. Finally, a clear case study of a bubble-free PMMA implant was obtained. | The manufacturing of CAD-designed molds with AM, replacing the production of the injury model, resulted in a 45% reduction in the manufacturing time of PMMA. Additionally, the implant showed a better fit than that produced by the conventional process. The use of AM molds for the manufacturing of PMMA implants has demonstrated a reduction in waiting time, which potentially can reduce patients' waiting time. | The joint use of CAD technology and FDM as an AM method to produce molds for PMMA implants for cranio-maxillofacial reconstruction seems to have many advantages, as it replaces several steps in the production of conventional PMMA implants, opening the possibility of minimizing dimensional errors due to handling. The use of polycarbonate molds manufactured with FDM for the direct manufacturing of PMMA implants, in the reported clinical case study, reduces the waiting time from 5 weeks (including delays in third-party services and the time needed for the patient to obtain economic resources) to 15 hours (a time reduction of 45.5% compared to the process currently used by HGM), which potentially can reduce the waiting time for patients being treated at the General Hospital of Mexico 'Dr. Eduardo Liceaga'. Furthermore, the use of MDF technology in medicine has been suggested beyond the manufacturing of medical models for visual representation and surgical planning. Although further research is needed to address the CAD of molds for more complex implants and determine the chemical properties of PMMA implants manufactured with this new methodology, it is possible to use FDM technology in medicine as an active tool for implant manufacturing. | Legal Aspects | Materials must be biocompatible, obtain patient consent for experiment. | PMMA was used for the implant and PC for the mold. For this study, the Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects, was followed. All patients were informed that their injuries would be used in this research and written informed consent was obtained. A 22-year-old male with a parietal-temporal lesion of 95 cm2 is the reported clinical case study. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Function | Heart: Stent that allows the passage of blood through a vessel or artery to the heart while degrading. Facilitate location during medical check-ups. | The first layer of the Absorb stent is composed of poly (L-lactide), which serves as the base polymer, and has the shape of a tube with zigzag rings connected by bridges. The next layer is a drug mixture, poly(D, L-lactide) (PDLLA), which serves as the drug-releasing layer, and then, on top, additional features are included such as a pair of radiopaque platinum markers that aim to assist in locating the stents during angiography and the balloon delivery system. | The implanted stent keeps the artery open, allowing blood to reach the heart. However, the placement of the stent in the coronary artery can cause injury to the vessel, triggering the formation of neointimal tissue inside the stent and an inflammatory response, leading to restenosis (recurrent narrowing of the coronary artery). As a result, repeated procedures are required, which are often associated with decreased patient well-being and increased healthcare costs (Kleinedler et al., 2012). To address this problem, advances have been made in the development of stents, including drug-eluting stents, polymeric stents, and stent manufacturing methods (Ramadugu and Latha Alikatte, 2016; Foerster et al., 2016). |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Function | Ears (External implants): headphone helmets retroauricular (BTE) or intraauricular (ITE) products | Design and manufacture of helmets by process with photosensitive resins, for the case of ITE customization is required that includes 3D scanning. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Function | Ears (internal implants): Graft of tympanic membrane (TM) that reproduces the specific structural characteristics of the human TM with acoustic properties that reflect the sound-induced movement patterns of the human TM and mechanical properties that demonstrate increased resistance to deformation compared to the temporal fascia. | They used multimaterial three-dimensional extrusion printing to design and manufacture a biomimetic TM graft in order to reproduce the specific structural characteristics of the human TM. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Function | Dental: Removable dental aligners (https://perfect-smile.cz/rovnatka-invisalign?gclid=EAIaIQobChMIgJKo8rOl6gIVzJ6zCh3augAeEAAYASAAEgI4avD_BwE) | Design and manufacture transparent removable and custom aligners through the 3D scanning process of the denture and manufacturing by SLA. | - |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Function | Throat: tracheal splint in the treatment of tracheobronchomalacia (TBM) in newborns (TBM is a disease that manifests with dynamic collapse of the airways and respiratory failure that is difficult to treat (Carden et al. 2005). | A splint was designed and manufactured in LS process material and PCL material with ductility to prevent particle fracture, and a 2-year stay time. | Long-term data showed continuous growth of the primary airways and all patients had significant improvements in their respiratory symptoms (Morrison et al. 2015). |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | aesthetic | External hearing aids: Select the color | Selection of skin colors but also black or other colors. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | aesthetic | Dental: Be transparent, be more comfortable. | Transparent material selection. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Customization | Heart: adjust the geometry of the stents, including the diameter, width, and thickness of the stent to the patient's geometry. | Part of imaging files, to deduce the main measurements to customize, and manufacture a custom implant. [2] | The majority of endoprostheses are available in various sizes and not always the size of the endoprosthesis fits the patient's vessel due to its geometry. 3D printing not only addresses the mentioned problems but also opens the possibility of producing personalized stents, which minimizes the probability of complications after stent transplantation and offers the possibility of controlling resistance by designing and printing stent struts with different thicknesses. |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Customization | Ears (External Implant): For headphone earmuffs, intra-auricular products (ITE). This last category is the most expensive as it has to be customized for each patient. | With over 1600 data points, biometric calibration takes into account the anatomy of your individual ear. Each Virto hearing aid is custom-made to provide a perfect and discreet fit and great comfort of use. (https://www.phonak.com/us/en.html) ME58 | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Customization | Dental: Each set must be customized to address specific patient issues. | We can determine in advance the possibilities of the digital world and the iTero® scanning. Each Invisalign ME59 treatment is individually planned for your case in order to achieve the best possible functional and aesthetic results. We carefully and precisely plan how quickly and in which direction your teeth will move and how they will look after treatment. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Customization | Throat | ||
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Dimensions | Heart: adjust the geometry of the stents, including the diameter, width, and thickness of the stent to the patient's geometry. | Part of imaging files, to deduce the main measurements to customize, and manufacture a custom implant. [2] | The majority of endoprostheses are available in various sizes and not always the size of the endoprosthesis fits the patient's vessel due to its geometry. 3D printing not only addresses the mentioned problems but also opens the possibility of producing personalized stents, which minimizes the probability of complications after stent transplantation and offers the possibility of controlling resistance by designing and printing stent struts with different thicknesses. |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Dimensions | Ears (external implant): For headphone earmuffs intra-auricular products (ITE) This last category is the most expensive as it has to be customized for each patient. | With over 1600 data points, biometric calibration takes into account the anatomy of your individual ear. Each Virto hearing aid is custom-made to provide a perfect and discreet fit and great comfort of use. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Material | Heart and Skin: biodegradable/biocompatible polymer that dissolves after 6 to 12 months leaving the vessel completely restored. The resulting mechanical properties should be similar to those of nitinol stents. | The most commonly used material for polymer-based stents is poly(L-lactide) (PLLA), which breaks down into absorbable lactic acid in the body over time. Other materials under development include polyglycolic acid, tyrosine, polycaprolactone, salicylic acid, or polycarbonate (Ang et al. 2017). They manufactured a cardiovascular stent using FDM. Computer-designed stents were fabricated using a commercially available copolyester polymer. | Currently there are few polymer-based stents available in the market, such as Absorb, Reva, and ReZolve. The most common stent, Absorb, produced by Abbott, offers an effective design of multiple components. The first layer of the Absorb stent is composed of poly(L-lactide), which serves as the base polymer, and has the shape of a tube with zigzag rings connected by bridges. The next layer is a drug mixture, poly(D, L-lactide) (PDLLA), which serves as the drug-releasing layer, and then on top, additional features are included such as a pair of radiopaque platinum markers that aim to assist in locating the stents during angiography and the balloon delivery system. |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Material | Ears (external implant): It must be a biocompatible material, It must be a strong, hard and water-resistant material like ABS. | While the LS seemed more promising as it used nylon. In 2002, a biocompatible material developed by Dreve materials in Germany suitable for SLA became available, and the hearing aid company Widex (https://www.widex.es/audifonos/tipos#formato) began implementing this technology. The material was a resin composed of a monomeric dimethacrylate, a urethane methacrylate, an aliphatic monomeric dimethacrylate, and the photoinitiator (Klare et al. 2004). Phonak (https://www.phonak.com/us/en.html) ME58 switched to using a tin polymerization method that uses digital light processing, a UV curable technology from EnvisionTEC. E-SHELL® 200 SERIES, E-SHELL® 300 SERIES, E-SHELL® 3000 SERIES, E-SHELL® 600: is a reactive liquid acrylate for the construction of functional parts, it is a low viscosity liquid photopolymer that produces strong, hard, and water-resistant ABS-like parts (https://envisiontec.com/3d-printing-industries/medical/hearing-aid/) Me60 | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Material | Ears (Internal Implant): with acoustic properties that reflect the patterns of movement induced by the sound of the human TM and mechanical properties that demonstrate greater resistance to deformation compared to the temporal fascia. | TM grafts were fabricated using polydimethylsiloxane (PDMS), flexible polylactic acid (PLA), and PCL materials followed by uniform filling with a hydrogel composed of fibrin and collagen. | The study demonstrated the feasibility of creating TM grafts with acoustic properties that reflect the motion patterns induced by human TM sound and mechanical properties that demonstrate increased resistance to deformation compared to temporal fascia. |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Material | Dental: It must be translucent and flexible, it must be biocompatible | The material used for the manufacture of these devices may include a styrenic block copolymer elastomer, a silicone rubber, an elastomeric alloy, a thermoplastic elastomer (TPE), a thermoplastic vulcanized elastomer (TPV), a polyurethane elastomer, a block copolymer elastomer, a blend of polyolefin elastomers, and a thermoplastic copolyester elastomer (Li and Chen 2012). | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Material | Throat: It must be biocompatible, it must have a long permanence time before reabsorption, it must be ductile in case of rupture not produce particles that perforate the tissue. | The implant was made using PCL with LS Technology. This material was selected because it has a long resorption time, which is very important in respiratory tract applications, as the implant must remain in place for at least 2 years before being absorbed. In addition, PCL is very ductile, so if it fails, it will not produce any particles that can puncture the tissue. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Manufacturing and assembly (production) | Heart: Tolerances must allow for patient adjustment, manufacturing must be compatible with bio-compatible and bio-absorbable materials. | Coronary stent made of biodegradable polylactic acid (PLLA) and PLLA-co-poly-e-caprolactone (PCL) using the laser sintering technique (SLM) for the first time. A post-processing step of spray coating and immersion coating has been included in the process to smooth the stent surface and allow for the incorporation of drugs in the future. Another 3D printing technique that has been investigated for stent manufacturing is extrusion. Park et al. (2015) successfully produced bioresorbable and drug-eluting PCL stents using this technique. Cabrera et al. (2017) fabricated a cardiovascular stent using FDM. Computer-designed stents were manufactured using a commercially available copolyester polymer that has been shown to biodegrade through hydrolysis. | After printing (extrusion), the stent was coated with the immunosuppressive drug followed by implantation in the artery and no complications were indicated. By optimizing the process parameters, it was possible to adjust the geometry of the stents, including the diameter, width, and thickness of the stent. The resulting mechanical properties were similar to those of nitinol stents. |
| GME6 | Implants | auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Manufacturing and assembly (production) | Ears (external implant): Reduce times and number of steps. | disposition a biocompatible material developed by Dreve materials in Germany suitable for SLA, and the hearing aid company Widex (https://www.widex.es/audifonos/tipos#formato) began implementing this technology. The material was a resin composed of a monomeric dimethacrylate, a urethane methacrylate, a monomeric aliphatic dimethacrylate, and the photoinitiator (Klare et al. 2004). Phonak (https://www.phonak. In the past, the manufacturing process of hearing aids consisted of more than nine steps that required more than a week of work. 3D printing has reduced the hearing aid manufacturing process to three steps: scanning, modeling, and printing.com/us/en.html) changed to use a tin polymerization method that uses digital light processing, a UV curable technology from EnvisionTEC | In the past, the process of manufacturing hearing aids consisted of more than nine steps that required over a week of work. 3D printing has reduced the hearing aid manufacturing process to three steps: scanning, modeling, and printing. This technology is capable of mass-producing hundreds of thousands of custom products each year. |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Manufacturing and assembly (production) | Ears (internal implant): It must be made of multiple materials to reproduce the acoustic and mechanical characteristics of the membrane. | They used multimaterial three-dimensional extrusion printing to design and manufacture a biomimetic TM graft in order to reproduce the specific structural characteristics of the human TM (Kozin et al. 2016). | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Manufacturing and assembly (production) | Dental: Sufficient production rates compared to traditional methods but combined with customization. | Uses digital dentistry and SLA technology to manufacture around 220,000 aligners per day, nearly 8 million per year (McCue 2017). | - |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Manufacturing and assembly (production) | Throat: It must be biocompatible. | The implant was made using PCL with LS Technology. This material was selected because it has a long resorption time, which is very important in respiratory tract applications, as the implant must remain in place for at least 2 years before being absorbed. In addition, PCL is very ductile, so if it fails, it will not produce any particles that can puncture the tissue. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Costs and deadlines | Ears (external implant): Reduce manufacturing times and deadlines compared to conventional processes. | Phonak (https://www.phonak.com/us/en.html) changed to use a tin polymerization method that uses digital light processing, a UV curable technology from EnvisionTEC. | In the past, the process of manufacturing hearing aids consisted of more than nine steps that required over a week of work. 3D printing has reduced the hearing aid manufacturing process to three steps: scanning, modeling, and printing. This technology is capable of mass-producing hundreds of thousands of custom products each year. |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Costs and deadlines | Increase production speed, reduce medical check-ups. Settling times similar to conventional ones. | Invisaling uses digital dentistry and SLA technology to manufacture around 220,000 aligners per day, almost 8 million per year (McCue 2017). Infrequent check-ups at the clinic. Usually once every 3 months. On average, Invisalign treatment lasts from 4 months to 1.5 years. It depends on the complexity of the case and, consequently, the type of Invisalign system used. Prices range from 2266 to 3690 USD. | A study conducted at Texas A&M University showed that there were no significant differences at any time between the two methods, indicating that lnvisalign and traditional appliances may have similar treatment outcomes, as well as similar settling during 6 months of retention (Preston 2017). |
| GME6 | Implants | auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Costs and deadlines | - | - | - |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Costs and deadlines | Throat: Implant must be maintained for two years. | since the implant must remain in place for at least 2 years before it is reabsorbed. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | Heart: It must be bio-compatible/bio-absorbable | Biodegradable coronary stent made of polylactic acid (PLLA) and PLLA-co-poly-e-caprolactone (PCL)/ copolyester polymer. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | Ears (external implant): Must be biocompatible. | It is certified by the EC and is biocompatible Class IIa according to ISO 10993 (Medical Products Law) for hearing aid covers and otoplastics. | - |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | - | - | - |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | Ears (Internal Implant): Must be biocompatible. | ||
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | Dental: It must be biocompatible. | Associated with selected material | - |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | Throat: It must be biocompatible. | The institutional examination board of the University of Michigan consulted with the Food and Drug Administration and approved the use of the device under the emergency use exemption, and the patient's parents provided their informed written consent. | It is necessary to continue working to optimize safe design and manufacturing processes within the framework of regulatory mandates and clinical trials (Shieh and Jennings 2017). |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | Heart: Must be bio-compatible/Bio-absorbable | Biodegradable coronary stent made of polylactic acid (PLLA) and PLLA-co-poly-e-caprolactone (PCL)/ copolyester polymer. | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | Ears (external implant): Must be biocompatible. | It is certified by the EC and is biocompatible Class IIa according to ISO 10993 (Medical Products Law) for hearing aid covers and otoplastics. | - |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | - | - | - |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | Ears (Internal Implant): Must be biocompatible. | ||
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | Dental: It must be biocompatible | Associated with selected material | |
| GME6 | Implants | Translated data: auditory implant, throat implant, nasal implant, cardiac implant, dental implant | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the structural design process for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | Throat: It must be biocompatible. | The institutional examination board of the University of Michigan consulted with the Food and Drug Administration and approved the use of the device under the emergency use exemption, and the patient's parents provided their informed written consent. | It is necessary to continue working to optimize safe design and manufacturing processes within the framework of regulatory mandates and clinical trials (Shieh and Jennings 2017). |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Function | Tissue Engineering/Ear Replacement/Trachea Replacement: Provide a bio-compatible and bio-absorbable, porous and interconnected environment for settlement, growth and irrigation of cells. | The synthetic polymers commonly used for scaffolds include absorbable types, such as PCL, PLA, and PEG, and non-absorbable types like polyurethane or polytetrafluoroethylene (PTFE). There are acellular scaffolds available in the market, for example, Alloderm DermACELL, FlexHD, or Integra, which have been shown to be effective scaffolds for wound healing, among others. | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Function | Tissue Engineering/Ear Replacement/Trachea Replacement: Provide a bio-compatible and bio-absorbable, porous and interconnected environment for settlement, growth and irrigation of cells. | The ear scaffold used a 3D printed polycaprolactone mesh as the inner core that was wrapped with non-woven PGA fibers and coated with PLA. | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Function | Tissue Engineering/Ear Replacement/Trachea Replacement: Provide a bio-compatible and bio-absorbable, porous and interconnected environment for settlement, growth and irrigation of cells. | 3D printed PCL scaffolds with a similar shape instead of the entire rabbit trachea | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Aesthetic | Replacement of ear: It must be aesthetic. | Computed tomography and 3D printing were used to guide the manufacturing of a biodegradable scaffold that replicated the exact symmetrical 3D structure of the healthy ear of the patient and had good mechanical properties. The ear scaffold used a 3D printed mesh. | Symmetry and tomography guarantee a personalized ear, similar to the original. A satisfactory aesthetic result was obtained with the formation of mature cartilage in the case performed in first (Zhou et al., 2018). |
| GME7 | Tissue engineering | tissue scaffold, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Customization/ Dimensions | Ear replacement: It must be identical to the patient's ear in shape and size. | Computed tomography and 3D printing were used to guide the manufacturing of a biodegradable scaffold that replicated the exact symmetrical 3D structure of the healthy ear of the patient and had good mechanical properties. The ear scaffold used a 3D printed mesh. | Symmetry and tomography guarantee a personalized ear, similar to the original. A satisfactory aesthetic result was obtained with the formation of mature cartilage in the case performed in first (Zhou et al., 2018). |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Material | Tissue engineering: Absorbable, bio-compatible with the cells to be cultivated. | The synthetic polymers commonly used for scaffolds include absorbable types, such as PCL, PLA, and PEG, and non-absorbable types like polyurethane or polytetrafluoroethylene (PTFE). There are acellular scaffolds available in the market, for example, Alloderm DermACELL, FlexHD, or Integra, which have been shown to be effective scaffolds for wound healing, among others. Although the advantages of synthetic polymers include relatively low cost and desired mechanical properties, their compatibility remains very low. The introduction of viable cells into polymeric scaffolds opens up the possibility of increasing material compatibility. Some of the most common cells used with cellular scaffolds include fibroblasts, keratinocytes, melanocytes, and stem cells. Cellular scaffolds available in the market. For example, it has been described that Apligraf and TransCyte function effectively for skin replacement therapy, among others. Despite extensive research, there is currently no ideal skin substitute available in the market. One of the reasons is the difficulty of replicating the complex multilayer structure of natural skin. | that have proven to be effective scaffolds for wound healing, among others. Although the advantages of synthetic polymers include a relatively low price and desired mechanical properties, their compatibility remains very low. |
| GME7 | Tissue engineering | tissue scaffold, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Material | Ear replacement: Absorbable, bio-compatible with the cells to be cultivated. | The ear scaffold used a 3D printed polycaprolactone mesh as the inner core, which was wrapped with non-woven PGA fibers and coated with PLA. After autologous chondrocytes derived from microtia cartilage were seeded onto the scaffold and cultured in vitro for 3 months, ear-shaped cartilage structures specific to each patient were generated and then implanted to reconstruct the auricles in living patients, with a longer follow-up time of 2.5 years. A satisfactory aesthetic outcome was achieved with the formation of mature cartilage in the case performed by Zhou et al. (2018). | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Material | Tracheal Replacement: Absorbable, bio-compatible with the cells to be cultivated. | After demonstrating the advantages of 3D printed splints, researchers are now investigating the possibility of seeding cells onto the devices to aid in cartilage formation during implantation. An in vivo study was conducted on animals to attempt long segmental repair of tracheal stenosis in rabbits. 3D printed PCL scaffolds with a similar shape to the rabbit's trachea were cultured with chondrocytes for 2 and 4 weeks prior to implantation to produce tissue-engineered trachea (TET). In order to evaluate the feasibility of repairing the entire trachea, the rabbit's trachea was surgically replaced with the TET. The study demonstrated that the TET cultured for 4 weeks had higher in vivo survival compared to the 2-week group, indicating that further in vitro maturation of the TET is needed for survival in the in vivo environment. | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Manufacturing and assembly (production) | Tissue engineering: - | SLA/SLM/LS/FDM/Multimaterial | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Costs and deadlines | Tissue engineering: maturation time and growth must ensure subsequent survival in implant. | ||
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Costs and deadlines | Ear replacement: maturation time and growth must ensure subsequent survival in implant. | They will be seeded onto the scaffold and cultured in vitro for 3 months, specific ear-shaped cartilage structures for each patient were generated and then implanted to reconstruct the auricles in living patients, with a longer follow-up time of 2.5 years. | Human experimentation and subsequent monitoring. |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Costs and deadlines | Tracheal replacement: maturation time and growth must ensure subsequent survival in implant. | The data translated into English is: Chondrocytes were cultured for 2 and 4 weeks before implantation to produce tissue-engineered trachea (TET). In order to evaluate the feasibility of whole trachea repair, the rabbit's trachea was surgically replaced with TET. The study demonstrated that TET cultured for 4 weeks had higher in vivo survival compared to 2 weeks, indicating that a longer in vitro maturation of TET is needed to survive in the in vivo environment. | Animal experimentation (trial and error) |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | Tissue Engineering/Ear Replacement/Trachea Replacement (portion): Must be biodegradable and bio-absorbable | There are acellular scaffolds available in the market, for example, Alloderm DermACELL, FlexHD or integra. | There are already commercial reference materials (there can be no sale unless legal requirements are met). |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | Tissue Engineering/Ear Replacement/Trachea Replacement (portion): Must be biodegradable and bio-absorbable | Polycaprolactone as an inner core that was wrapped with non-woven PGA fibers and coated with PLA. After autologous chondrocytes derived from microtia cartilage were seeded on the scaffold and cultured in vitro for 3 months. | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Security | Tissue Engineering/Ear Replacement/Trachea Replacement (portion): Must be biodegradable and bio-absorbable | . 3D printed PCL scaffolds with a similar shape instead of the entire rabbit trachea were cultured with chondrocytes for 2 and 4 weeks before implantation to produce tissue-engineered trachea (TET). | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | Tissue Engineering/Ear Replacement/Trachea Replacement (portion): Must be biodegradable and bio-absorbable | There are acellular scaffolds available in the market, for example, Alloderm DermACELL, FlexHD or integra. | There are already commercial reference materials (there can be no sale unless legal requirements are met). |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | Tissue Engineering/Ear Replacement/Trachea Replacement (portion): Must be biodegradable and bio-absorbable | Poly-ε-caprolactone as an inner core was wrapped with PGA nonwoven fibers and coated with PLA. After autologous chondrocytes derived from microtia cartilage were seeded onto the scaffold and cultured in vitro for 3 months. | |
| GME7 | Tissue engineering | tissue scaffolding, tissue implant (ear replacement, trachea replacement) | ME56, ME57, | A vision of the global market for 3D printed medical devices is presented, along with a more detailed discussion of different applications, including artificial skin: cardiovascular stents; otorhinolaryngology and dentistry. The chapter examines recent advances in 3D printing technologies for biomedical implants. The latest advances in 3D printing of artificial skin are described, with a focus on the treatment of burn wounds. The chapter also examines the methods and materials being used for 3D printing of stents, including computer-printed metal, biodegradable polymers, self-expanding stents, and drug-eluting stents. Otorhinolaryngology is a specialized area where AM has also been successfully used for implant manufacturing. Examples of implants, such as hearing aids that are already on the market, and 3D printing techniques that can be used to create implants to replace middle ear bones (ossicles) when damaged or to reconstruct the entire ear, are examined. Finally, dental implants in the research and development stage are reviewed. | Literature review | The application of 3D printing for the manufacturing of skin substitutes for wound treatment has proven to be effective and has accelerated the healing processes. The ability to create multiple complex geometries and use a wide range of biomaterials and cells has great value for skin manufacturing. The technological progression of 3D printing can further improve wound healing by creating larger skin patches and covering wider areas of the wounds with the hope of being used in future clinical trials. New advancements will require new biomaterials, optimization of scaffold design, and a better understanding of cell functioning. 3D printing technology is also a promising and developing approach for polymer processing and manufacturing of cardiovascular stents. The application of 3D printing aids in the design process of structures for stent manufacturing, ultimately influencing the technical properties of the stents. Biocompatible materials with the right balance between mechanical properties and degradation rate are still needed. The fields of otorhinolaryngology and dentistry were the first to implement 3D technology in their manufacturing methods; this technology has allowed them to produce customized hearing aids and dental aligners in a serial production. In addition to these two medical devices already on the market, there are several 3D printed implants such as the trachea, ear, and nose replacements that have already been implanted in humans and have shown promising results. Clinical trials are currently being conducted to demonstrate the safety and efficacy of these implants in order to comply with all regulations. | Although the application of 3D printing in the biomedical field has reached the market for the aforementioned applications, the technology is still in the development process. Ongoing research seems promising to make it used as a standard manufacturing method in both clinics and industry. | Legal Aspects | Tissue Engineering/Ear Replacement/Trachea Replacement (portion): Must be biodegradable and bio-absorbable | . 3D printed PCL scaffolds with a similar shape instead of the entire rabbit trachea were cultured with chondrocytes for 2 and 4 weeks before implantation to produce tissue-engineered trachea (TET). | |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME8 | Here is the translated data in English: We present a new technique for manufacturing double-pore scaffolds for various tissue engineering applications, in which 3D printing of poly(vinyl alcohol) (PVA) filament mold is combined with salt leaching process to control the geometry and dimensions of structured and random pores, respectively. | The sacrifice molds for structured and double-pore scaffolds were 3D printed using a Replicator 2X 3D filament deposition printer (MakerBot Industries, LLC, Brooklyn, NY, USA). In summary, a 3D cube (L 25 mm × W 25 mm × H 10 mm) was designed using SolidWorks 2013 CAD 3D design software (Dassault Systems, SolidWorks Corp., Waltham, MA, USA). The design was exported in STL format to the 3D printing software (Makerware 2.4.1) for G-code generation and mold printing. Poly(vinyl alcohol) (PVA) soluble in water filaments (MakerBot, USA) were used to print the sacrificial molds. The printing was done at 200 °C nozzle temperature and 40 °C bed temperature. PVA molds with various porosities (fillers) were used directly for the fabrication of structured pore scaffolds. For the fabrication of double-pore scaffolds, PVA molds were printed with 40% filler. 10 g of NaCl crystals (Sigma-Aldrich Corporation, St. Louis, MO, USA) (size 300-600 μm) were mixed with 1 ml of ultrapure water from a Milli-Q® water purification system (Millipore Corporation, Billerica, MA, USA) and manually packed into the PVA mold. The salt-filled molds were dried in an oven at 60 °C for 1 h. The small amount of water was used to form interconnections between the salt crystals, as well as between the salt crystals and the PVA filaments. Both PVA molds for structured pore scaffolds and salt-PVA molds for double-pore scaffolds were replicated in polydimethylsiloxane (PDMS) (Sylgard 184 elastomer kit, Dow Corning Corporation, Midland, MI, USA). The PDMS base was thoroughly mixed with the curing agent in a ratio of 10:1 and the mixture was degassed in a vacuum desiccator to remove air bubbles. The PDMS mixture was then poured into the mold, and the container with the mold covered with the PDMS mixture was transferred to the vacuum desiccator for 2-3 h to ensure complete infiltration of PDMS into the mold. The PDMS was cured at 60 °C in an oven for 4-6 h. After curing, the excess PDMS around the molds was removed to expose the PVA or salt-PVA structures. This was done to allow subsequent dissolution of PVA and salt in water. The complete PDMS structures of the molds were immersed in water until the PVA and salt were completely removed (around 6-12 h), releasing the PDMS scaffolds with structured or double-pore architecture. Various physical and mechanical properties of the double-pore scaffolds were compared with control scaffolds with only structured or only random pores, fabricated using previously reported methods. | The scaffolds with double pores showed intermediate mechanical properties compared to scaffolds with structured and random pores. The compression modulus of the structured pore scaffolds was similar to that of our previous study presented with similar type scaffolds. The result of the mechanical test indicates that the mechanical properties of the double pore scaffolds can be easily modified by combining random and structured pores. Since the elastic moduli of human organs and tissues are very diverse, the stiffness of the fabricated double pore PDMS scaffolds can be adjusted by changing the porosity of the scaffolds or the degree of crosslinking, i.e., the ratio between the PDMS base and the curing agent [36]. As mentioned earlier, the manufacturing technique presented in this document can be applied to other materials besides PDMS, such as the biomaterial PCL (supplementary material Fig. S5), which provides an additional way to fine-tune the stiffness of the scaffolds according to the needs of in vivo tissues. The double pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. | A method for manufacturing double-pore scaffolds by combining 3D filament printing of a water-soluble sacrificial PVA mold for the primary structured pores and salt leaching to create random pores within the bulk scaffold. The method allows for control of pore geometry and dimensions, as well as the mechanical stiffness of the structured and random pores of the double-pore scaffold. Using PDMS as the scaffold material, the manufacturing process was demonstrated to be fast, inexpensive, and scalable for generating scaffolds of a size relevant to thick tissues. Sacrificial molds printed with PVA can also be used with different biodegradable natural polymers (e.g., gelatin and silk) combined with lyophilization to provide scaffolds with a wide range of mechanical properties. Human hepatoblastoma cells (HepG2) were cultured on different types of scaffolds for 16 days, monitoring their adhesion, distribution, proliferation, viability, metabolic activity, and albumin secretion, and cells were cultured on double-pore scaffolds with cells cultured on scaffolds with only structured or only random pores. The double-pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. In conclusion, the described manufacturing technique is fast, inexpensive, scalable, and compatible with different polymers, making it suitable for engineering various organs/tissues on a large scale. | Function | Manufacture double-pore scaffolding for tissue engineering, with controllable pore size, uniform and random distribution, and interconnection, all in as automated a process as possible. | 3D printing of a poly(vinyl alcohol) (PVA) mold is combined with the salt leaching process. | In this technique, the sacrificial PVA mold, which determines the architecture of the structured pores, was filled with salt crystals to define the regions of random pores of the scaffold. After crosslinking the melted polymer, the combined PVA and salt mold was dissolved in water. The dual-pore scaffold is superior to the other two types of scaffolds as it combines a high number of cells (Fig. 5A), metabolic activity (Fig. 5B), and albumin secretion (Fig. 5C) with a uniform distribution of live cells both in (Fig. 6C) and in the scaffolds (Fig. 7C). |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME8 | Here is the translated data in English: We present a new technique for manufacturing double-pore scaffolds for various tissue engineering applications, in which 3D printing of poly(vinyl alcohol) (PVA) filament mold is combined with salt leaching process to control the geometry and dimensions of structured and random pores, respectively. | The sacrifice molds for structured and double-pore scaffolds were 3D printed using a Replicator 2X 3D filament deposition printer (MakerBot Industries, LLC, Brooklyn, NY, USA). In summary, a 3D cube (L 25 mm × W 25 mm × H 10 mm) was designed using SolidWorks 2013 CAD 3D design software (Dassault Systems, SolidWorks Corp., Waltham, MA, USA). The design was exported in STL format to the 3D printing software (Makerware 2.4.1) for G-code generation and mold printing. Poly(vinyl alcohol) (PVA) soluble in water filaments (MakerBot, USA) were used to print the sacrificial molds. The printing was done at 200 °C nozzle temperature and 40 °C bed temperature. PVA molds with various porosities (fillers) were used directly for the fabrication of structured pore scaffolds. For the fabrication of double-pore scaffolds, PVA molds were printed with 40% filler. 10 g of NaCl crystals (Sigma-Aldrich Corporation, St. Louis, MO, USA) (size 300-600 μm) were mixed with 1 ml of ultrapure water from a Milli-Q® water purification system (Millipore Corporation, Billerica, MA, USA) and manually packed into the PVA mold. The salt-filled molds were dried in an oven at 60 °C for 1 h. The small amount of water was used to form interconnections between the salt crystals, as well as between the salt crystals and the PVA filaments. Both PVA molds for structured pore scaffolds and salt-PVA molds for double-pore scaffolds were replicated in polydimethylsiloxane (PDMS) (Sylgard 184 elastomer kit, Dow Corning Corporation, Midland, MI, USA). The PDMS base was thoroughly mixed with the curing agent in a ratio of 10:1 and the mixture was degassed in a vacuum desiccator to remove air bubbles. The PDMS mixture was then poured into the mold, and the container with the mold covered with the PDMS mixture was transferred to the vacuum desiccator for 2-3 h to ensure complete infiltration of PDMS into the mold. The PDMS was cured at 60 °C in an oven for 4-6 h. After curing, the excess PDMS around the molds was removed to expose the PVA or salt-PVA structures. This was done to allow subsequent dissolution of PVA and salt in water. The complete PDMS structures of the molds were immersed in water until the PVA and salt were completely removed (around 6-12 h), releasing the PDMS scaffolds with structured or double-pore architecture. Various physical and mechanical properties of the double-pore scaffolds were compared with control scaffolds with only structured or only random pores, fabricated using previously reported methods. | The scaffolds with double pores showed intermediate mechanical properties compared to scaffolds with structured and random pores. The compression modulus of the structured pore scaffolds was similar to that of our previous study presented with similar type scaffolds. The result of the mechanical test indicates that the mechanical properties of the double pore scaffolds can be easily modified by combining random and structured pores. Since the elastic moduli of human organs and tissues are highly diverse, the stiffness of the fabricated double pore PDMS scaffolds can be adjusted by changing the porosity of the scaffolds or the degree of crosslinking, i.e., the ratio between PDMS base and curing agent [36]. As mentioned earlier, the manufacturing technique presented in this document can be applied to other materials besides PDMS, such as the biomaterial PCL (supplementary material Fig. S5), which provides an additional way to fine-tune the stiffness of the scaffolds according to the needs of in vivo tissues. The double pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. | A method for manufacturing double-pore scaffolds by combining 3D filament printing of a water-soluble sacrificial PVA mold for the primary structured pores and salt leaching to create random pores within the bulk scaffold. The method allows for control of pore geometry and dimensions, as well as the mechanical stiffness of the structured and random pores of the double-pore scaffold. Using PDMS as the scaffold material, the manufacturing process was demonstrated to be fast, inexpensive, and scalable for generating scaffolds of a size relevant to thick tissues. Sacrificial molds printed with PVA can also be used with different biodegradable natural polymers (e.g., gelatin and silk) combined with lyophilization to provide scaffolds with a wide range of mechanical properties. Human hepatoblastoma cells (HepG2) were cultured on different types of scaffolds for 16 days, monitoring their adhesion, distribution, proliferation, viability, metabolic activity, and albumin secretion, and cells were cultured on double-pore scaffolds with cells cultured on scaffolds with only structured or only random pores. The double-pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. In conclusion, the described manufacturing technique is fast, inexpensive, scalable, and compatible with different polymers, making it suitable for engineering various organs/tissues on a large scale. | Dimensions | Distances of channels ranging from 78 μm to 1482 μm, interconnected pores, pores of size between 300-600 um. | A cube was designed in 3D (L 25 mm × W 25 mm × H 10 mm) using the CAD 3D design software SolidWorks 2013 (Dassault Systems, SolidWorks Corp., Waltham, MA, USA). PVA molds printed with various porosities (fillers) were directly used for the fabrication of structured pore scaffolds. For the fabrication of double-pore scaffolds, PVA molds were printed with a 40% filler, 10 g of NaCl crystals (Sigma-Aldrich Corporation, St. Louis, MO, USA) (size 300-600 μm) were mixed with 1 ml of ultrapure water from a Milli-Q® water purification system (Millipore Corporation, Billerica, MA, USA) and manually packed into the PVA mold (Fig. 1B). Therefore, to generate structured pores for double-pore scaffolds, 3D printing of PVA filaments was an obvious choice for our manufacturing process presented here. In this work, a 40% filler was used to fabricate PVA molds for double-pore scaffolds, resulting in a filament spacing of 800-850 μm. Leaching of salt as a source of random pores provides the possibility to control the range of pore sizes by choosing the size distribution of salt crystals to mimic the anatomical features of tissues or organs required for their engineering [26]. In this study, NaCl crystals were directly used as supplied by the supplier, with sizes ranging from 300 μm to 600 μm. | The PVA molds could be manufactured with porosities in the range of 20-80% depending on the mold filling, which was shown to correspond to channel distances ranging from 78 μm to 1482 μm [25]. As shown in the SEM image of the structured pore scaffold in Fig. 2Ac, the pores have a square shape with a dimension of ~400 μm × 400 μm and are well interconnected. In the random pore scaffolds, the salt leaching process results in pores with dimensions in the range of 300-600 μm (Fig. 2Bc), as determined by the size distribution of the salt crystals used. The scaffolds had regular, well-structured, and highly interconnected structured pores. Among the structured pores, the scaffolds had a set of randomly porous regions with a square shape of 800 μm × 800 μm. Salt crystals of size 300-600 μm were fused and generated large interconnected pores. The scaffold channels have an elliptical cross-sectional profile, as the round PVA filament is flattened during mold printing, and the distance between two rows of structured pores is approximately 800 μm. The pore dimensions (width 344 μm, height 190 μm) are slightly smaller than those of the printed mold filaments (400 μm × 200 μm), due to shrinkage during PDMS elastomer curing. A region of random pores can be seen in the area between two rows of structured pores (blue dashed line). To demonstrate the possibility of controlling the size of random pores, the above-described PVA mold was filled with smaller salt or sugar crystals, resulting in clearly defined highly interconnected small random pores (20-50 μm) along with the structured pores of 400 μm × 400 μm. The distance between the filaments of a PVA mold needs to be optimized according to the size of the salt or sugar crystals required to generate the random pores. Using different sizes of porous mesh, it is possible to obtain salt or sugar crystals with different size distribution. If large crystals are required, the distance between the deposited PVA filaments must be increased, reducing the porosity of the PVA mold from 40% filling to less. |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME8 | Here is the translated data in English: We present a new technique for manufacturing double-pore scaffolds for various tissue engineering applications, in which 3D printing of poly(vinyl alcohol) (PVA) filament mold is combined with salt leaching process to control the geometry and dimensions of structured and random pores, respectively. | The sacrifice molds for structured and double-pore scaffolds were 3D printed using a Replicator 2X 3D filament deposition printer (MakerBot Industries, LLC, Brooklyn, NY, USA). In summary, a 3D cube (L 25 mm × W 25 mm × H 10 mm) was designed using SolidWorks 2013 CAD 3D design software (Dassault Systems, SolidWorks Corp., Waltham, MA, USA). The design was exported in STL format to the 3D printing software (Makerware 2.4.1) for G-code generation and mold printing. Poly(vinyl alcohol) (PVA) soluble in water filaments (MakerBot, USA) were used to print the sacrificial molds. The printing was done at 200 °C nozzle temperature and 40 °C bed temperature. PVA molds with various porosities (fillers) were used directly for the fabrication of structured pore scaffolds. For the fabrication of double-pore scaffolds, PVA molds were printed with 40% filler. 10 g of NaCl crystals (Sigma-Aldrich Corporation, St. Louis, MO, USA) (size 300-600 μm) were mixed with 1 ml of ultrapure water from a Milli-Q® water purification system (Millipore Corporation, Billerica, MA, USA) and manually packed into the PVA mold. The salt-filled molds were dried in an oven at 60 °C for 1 h. The small amount of water was used to form interconnections between the salt crystals, as well as between the salt crystals and the PVA filaments. Both PVA molds for structured pore scaffolds and salt-PVA molds for double-pore scaffolds were replicated in polydimethylsiloxane (PDMS) (Sylgard 184 elastomer kit, Dow Corning Corporation, Midland, MI, USA). The PDMS base was thoroughly mixed with the curing agent in a ratio of 10:1 and the mixture was degassed in a vacuum desiccator to remove air bubbles. The PDMS mixture was then poured into the mold, and the container with the mold covered with the PDMS mixture was transferred to the vacuum desiccator for 2-3 h to ensure complete infiltration of PDMS into the mold. The PDMS was cured at 60 °C in an oven for 4-6 h. After curing, the excess PDMS around the molds was removed to expose the PVA or salt-PVA structures. This was done to allow subsequent dissolution of PVA and salt in water. The complete PDMS structures of the molds were immersed in water until the PVA and salt were completely removed (around 6-12 h), releasing the PDMS scaffolds with structured or double-pore architecture. Various physical and mechanical properties of the double-pore scaffolds were compared with control scaffolds with only structured or only random pores, fabricated using previously reported methods. | The scaffolds with double pores showed intermediate mechanical properties compared to scaffolds with structured and random pores. The compression modulus of the structured pore scaffolds was similar to that of our previous study presented with similar type scaffolds. The result of the mechanical test indicates that the mechanical properties of the double pore scaffolds can be easily modified by combining random and structured pores. Since the elastic moduli of human organs and tissues are highly diverse, the stiffness of the fabricated double pore PDMS scaffolds can be adjusted by changing the porosity of the scaffolds or the degree of crosslinking, i.e., the ratio between PDMS base and curing agent [36]. As mentioned earlier, the manufacturing technique presented in this document can be applied to other materials besides PDMS, such as the biomaterial PCL (supplementary material Fig. S5), which provides an additional way to fine-tune the stiffness of the scaffolds according to the needs of in vivo tissues. The double pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. | A method for manufacturing double-pore scaffolds by combining 3D filament printing of a water-soluble sacrificial PVA mold for the primary structured pores and salt leaching to create random pores within the bulk scaffold. The method allows for control of pore geometry and dimensions, as well as the mechanical stiffness of the structured and random pores of the double-pore scaffold. Using PDMS as the scaffold material, the manufacturing process was demonstrated to be fast, inexpensive, and scalable for generating scaffolds of a size relevant to thick tissues. Sacrificial molds printed with PVA can also be used with different biodegradable natural polymers (e.g., gelatin and silk) combined with lyophilization to provide scaffolds with a wide range of mechanical properties. Human hepatoblastoma cells (HepG2) were cultured on different types of scaffolds for 16 days, monitoring their adhesion, distribution, proliferation, viability, metabolic activity, and albumin secretion, and cells were cultured on double-pore scaffolds with cells cultured on scaffolds with only structured or only random pores. The double-pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. In conclusion, the described manufacturing technique is fast, inexpensive, scalable, and compatible with different polymers, making it suitable for engineering various organs/tissues on a large scale. | Material | The final material must be biocompatible, it must also have a rigidity according to the tissues where it will be implanted. | PVA, Water, Salt, PDMS | PDMS is biocompatible, to achieve the shape a printed mold and salt grains are used. It has been previously shown that the compression modulus of porous scaffolds is significantly reduced as their porosity increases. For the same reason, double-pore scaffolds exhibited intermediate mechanical properties compared to structured and random pore scaffolds, indicating the expected relationship between porosity and mechanical stiffness of the scaffolds. The compression modulus of structured pore scaffolds was similar to that of our previous study presented with similar type scaffolds (about 360 kPa). Furthermore, our mechanical tests on the different scaffolds showed a similar trend as previously reported for structured, random, and double-pore scaffolds made with PCL. The result of the mechanical test indicates that the mechanical properties of double-pore scaffolds can be easily modified by combining random and structured pores. Since the elastic moduli of human organs and tissues are highly diverse, the stiffness of the manufactured double-pore PDMS scaffolds can be adjusted by changing the porosity of the scaffolds or the degree of crosslinking, i.e., the ratio between PDMS base and curing agent. As mentioned earlier, the manufacturing technique presented in this document can be applied to other materials besides PDMS, such as the biomaterial PCL (supplementary material Fig. S5), which provides an additional way to fine-tune the stiffness of the scaffolds according to the needs of in vivo tissues. |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME8 | Here is the translated data in English: We present a new technique for manufacturing double-pore scaffolds for various tissue engineering applications, in which 3D printing of poly(vinyl alcohol) (PVA) filament mold is combined with salt leaching process to control the geometry and dimensions of structured and random pores, respectively. | The sacrifice molds for structured and double-pore scaffolds were 3D printed using a Replicator 2X 3D filament deposition printer (MakerBot Industries, LLC, Brooklyn, NY, USA). In summary, a 3D cube (L 25 mm × W 25 mm × H 10 mm) was designed using SolidWorks 2013 CAD 3D design software (Dassault Systems, SolidWorks Corp., Waltham, MA, USA). The design was exported in STL format to the 3D printing software (Makerware 2.4.1) for G-code generation and mold printing. Poly(vinyl alcohol) (PVA) soluble in water filaments (MakerBot, USA) were used to print the sacrificial molds. The printing was done at 200 °C nozzle temperature and 40 °C bed temperature. PVA molds with various porosities (fillers) were used directly for the fabrication of structured pore scaffolds. For the fabrication of double-pore scaffolds, PVA molds were printed with 40% filler. 10 g of NaCl crystals (Sigma-Aldrich Corporation, St. Louis, MO, USA) (size 300-600 μm) were mixed with 1 ml of ultrapure water from a Milli-Q® water purification system (Millipore Corporation, Billerica, MA, USA) and manually packed into the PVA mold. The salt-filled molds were dried in an oven at 60 °C for 1 h. The small amount of water was used to form interconnections between the salt crystals, as well as between the salt crystals and the PVA filaments. Both PVA molds for structured pore scaffolds and salt-PVA molds for double-pore scaffolds were replicated in polydimethylsiloxane (PDMS) (Sylgard 184 elastomer kit, Dow Corning Corporation, Midland, MI, USA). The PDMS base was thoroughly mixed with the curing agent in a ratio of 10:1 and the mixture was degassed in a vacuum desiccator to remove air bubbles. The PDMS mixture was then poured into the mold, and the container with the mold covered with the PDMS mixture was transferred to the vacuum desiccator for 2-3 h to ensure complete infiltration of PDMS into the mold. The PDMS was cured at 60 °C in an oven for 4-6 h. After curing, the excess PDMS around the molds was removed to expose the PVA or salt-PVA structures. This was done to allow subsequent dissolution of PVA and salt in water. The complete PDMS structures of the molds were immersed in water until the PVA and salt were completely removed (around 6-12 h), releasing the PDMS scaffolds with structured or double-pore architecture. Various physical and mechanical properties of the double-pore scaffolds were compared with control scaffolds with only structured or only random pores, fabricated using previously reported methods. | The scaffolds with double pores showed intermediate mechanical properties compared to scaffolds with structured and random pores. The compression modulus of the structured pore scaffolds was similar to that of our previous study presented with similar type scaffolds. The result of the mechanical test indicates that the mechanical properties of the double pore scaffolds can be easily modified by combining random and structured pores. Since the elastic moduli of human organs and tissues are highly diverse, the stiffness of the fabricated double pore PDMS scaffolds can be adjusted by changing the porosity of the scaffolds or the degree of crosslinking, i.e., the ratio between PDMS base and curing agent [36]. As mentioned earlier, the manufacturing technique presented in this document can be applied to other materials besides PDMS, such as the biomaterial PCL (supplementary material Fig. S5), which provides an additional way to fine-tune the stiffness of the scaffolds according to the needs of in vivo tissues. The double pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. | A method for manufacturing double-pore scaffolds by combining 3D filament printing of a water-soluble sacrificial PVA mold for the primary structured pores and salt leaching to create random pores within the bulk scaffold. The method allows for control of pore geometry and dimensions, as well as the mechanical stiffness of the structured and random pores of the double-pore scaffold. Using PDMS as the scaffold material, the manufacturing process was demonstrated to be fast, inexpensive, and scalable for generating scaffolds of a size relevant to thick tissues. Sacrificial molds printed with PVA can also be used with different biodegradable natural polymers (e.g., gelatin and silk) combined with lyophilization to provide scaffolds with a wide range of mechanical properties. Human hepatoblastoma cells (HepG2) were cultured on different types of scaffolds for 16 days, monitoring their adhesion, distribution, proliferation, viability, metabolic activity, and albumin secretion, and cells were cultured on double-pore scaffolds with cells cultured on scaffolds with only structured or only random pores. The double-pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. In conclusion, the described manufacturing technique is fast, inexpensive, scalable, and compatible with different polymers, making it suitable for engineering various organs/tissues on a large scale. | Manufacturing and Assembly | The process must combine conventional methods with 3D printing to achieve manufacturing in a biocompatible material with controlled sizes of pores and channels. | Positive mold is made using FFF, PVA material, water, and salt grains. Later, PDMS and water are used to sacrifice the PVA. | To print the sacrifice molds, water-soluble poly(vinyl alcohol) (PVA) filaments were used (MakerBot, USA). Printing was done at 200 °C as the nozzle temperature and 40 °C as the bed temperature. PVA molds printed with various porosities (fillers) were directly used for the fabrication of structured pore scaffolds. For the fabrication of double-pore scaffolds, PVA molds were printed with a 40% filler. 10 g of NaCl crystals (Sigma-Aldrich Corporation, St. Louis, MO, USA) (size 300-600 μm) were mixed with 1 ml of ultrapure water from a Milli-Q® water purification system (Millipore Corporation, Billerica, MA, USA) and manually packed into the PVA mold. The salt-filled molds were dried in an oven at 60 °C for 1 h. The small amount of water was used to form the interconnections between the salt crystals, as well as between the salt crystals and the PVA filaments. Both the PVA molds for structured pore scaffolds and the salt-PVA molds for double-pore scaffolds were replicated in polydimethylsiloxane (PDMS) (Sylgard 184 elastomer kit, Dow Corning Corporation, Midland, MI, USA). The PDMS base was thoroughly mixed with the curing agent in a ratio of 10:1 and the mixture was degassed in a vacuum desiccator to remove air bubbles. The PDMS mixture was then poured into the mold, and the container with the mold covered with the PDMS mixture was transferred to the vacuum desiccator for 2-3 h to ensure complete infiltration of PDMS into the mold. The PDMS was cured at 60 °C in an oven for 4-6 h. After curing, the excess PDMS around the molds was removed to expose the PVA or salt-PVA structures. This was done to allow for the subsequent dissolution of PVA and salt in water. The complete PDMS structures of the molds were immersed in water until the PVA and salt were completely removed (around 6-12 h), releasing the PDMS scaffolds with structured pore or double-pore architecture. |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME8 | Here is the translated data in English: We present a new technique for manufacturing double-pore scaffolds for various tissue engineering applications, in which 3D printing of poly(vinyl alcohol) (PVA) filament mold is combined with salt leaching process to control the geometry and dimensions of structured and random pores, respectively. | The sacrifice molds for structured and double-pore scaffolds were 3D printed using a Replicator 2X 3D filament deposition printer (MakerBot Industries, LLC, Brooklyn, NY, USA). In summary, a 3D cube (L 25 mm × W 25 mm × H 10 mm) was designed using SolidWorks 2013 CAD 3D design software (Dassault Systems, SolidWorks Corp., Waltham, MA, USA). The design was exported in STL format to the 3D printing software (Makerware 2.4.1) for G-code generation and mold printing. Poly(vinyl alcohol) (PVA) soluble in water filaments (MakerBot, USA) were used to print the sacrificial molds. The printing was done at 200 °C nozzle temperature and 40 °C bed temperature. PVA molds with various porosities (fillers) were used directly for the fabrication of structured pore scaffolds. For the fabrication of double-pore scaffolds, PVA molds were printed with 40% filler. 10 g of NaCl crystals (Sigma-Aldrich Corporation, St. Louis, MO, USA) (size 300-600 μm) were mixed with 1 ml of ultrapure water from a Milli-Q® water purification system (Millipore Corporation, Billerica, MA, USA) and manually packed into the PVA mold. The salt-filled molds were dried in an oven at 60 °C for 1 h. The small amount of water was used to form interconnections between the salt crystals, as well as between the salt crystals and the PVA filaments. Both PVA molds for structured pore scaffolds and salt-PVA molds for double-pore scaffolds were replicated in polydimethylsiloxane (PDMS) (Sylgard 184 elastomer kit, Dow Corning Corporation, Midland, MI, USA). The PDMS base was thoroughly mixed with the curing agent in a ratio of 10:1 and the mixture was degassed in a vacuum desiccator to remove air bubbles. The PDMS mixture was then poured into the mold, and the container with the mold covered with the PDMS mixture was transferred to the vacuum desiccator for 2-3 h to ensure complete infiltration of PDMS into the mold. The PDMS was cured at 60 °C in an oven for 4-6 h. After curing, the excess PDMS around the molds was removed to expose the PVA or salt-PVA structures. This was done to allow subsequent dissolution of PVA and salt in water. The complete PDMS structures of the molds were immersed in water until the PVA and salt were completely removed (around 6-12 h), releasing the PDMS scaffolds with structured or double-pore architecture. Various physical and mechanical properties of the double-pore scaffolds were compared with control scaffolds with only structured or only random pores, fabricated using previously reported methods. | The scaffolds with double pores showed intermediate mechanical properties compared to scaffolds with structured and random pores. The compression modulus of the structured pore scaffolds was similar to that of our previous study presented with similar type scaffolds. The result of the mechanical test indicates that the mechanical properties of the double pore scaffolds can be easily modified by combining random and structured pores. Since the elastic moduli of human organs and tissues are highly diverse, the stiffness of the fabricated double pore PDMS scaffolds can be adjusted by changing the porosity of the scaffolds or the degree of crosslinking, i.e., the ratio between PDMS base and curing agent [36]. As mentioned earlier, the manufacturing technique presented in this document can be applied to other materials besides PDMS, such as the biomaterial PCL (supplementary material Fig. S5), which provides an additional way to fine-tune the stiffness of the scaffolds according to the needs of in vivo tissues. The double pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. | A method for manufacturing double-pore scaffolds by combining 3D filament printing of a water-soluble sacrificial PVA mold for the primary structured pores and salt leaching to create random pores within the bulk scaffold. The method allows for control of pore geometry and dimensions, as well as the mechanical stiffness of the structured and random pores of the double-pore scaffold. Using PDMS as the scaffold material, the manufacturing process was demonstrated to be fast, inexpensive, and scalable for generating scaffolds of a size relevant to thick tissues. Sacrificial molds printed with PVA can also be used with different biodegradable natural polymers (e.g., gelatin and silk) combined with lyophilization to provide scaffolds with a wide range of mechanical properties. Human hepatoblastoma cells (HepG2) were cultured on different types of scaffolds for 16 days, monitoring their adhesion, distribution, proliferation, viability, metabolic activity, and albumin secretion, and cells were cultured on double-pore scaffolds with cells cultured on scaffolds with only structured or only random pores. The double-pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. In conclusion, the described manufacturing technique is fast, inexpensive, scalable, and compatible with different polymers, making it suitable for engineering various organs/tissues on a large scale. | Security | It must be bio-compatible. | PDMS is chosen as the material for the final scaffold. | In the United States, the FDA approves the use of dimethicone as safe, as it has been widely used for many years. |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME8 | Here is the translated data in English: We present a new technique for manufacturing double-pore scaffolds for various tissue engineering applications, in which 3D printing of poly(vinyl alcohol) (PVA) filament mold is combined with salt leaching process to control the geometry and dimensions of structured and random pores, respectively. | The sacrifice molds for structured and double-pore scaffolds were 3D printed using a Replicator 2X 3D filament deposition printer (MakerBot Industries, LLC, Brooklyn, NY, USA). In summary, a 3D cube (L 25 mm × W 25 mm × H 10 mm) was designed using SolidWorks 2013 CAD 3D design software (Dassault Systems, SolidWorks Corp., Waltham, MA, USA). The design was exported in STL format to the 3D printing software (Makerware 2.4.1) for G-code generation and mold printing. Poly(vinyl alcohol) (PVA) soluble in water filaments (MakerBot, USA) were used to print the sacrificial molds. The printing was done at 200 °C nozzle temperature and 40 °C bed temperature. PVA molds with various porosities (fillers) were used directly for the fabrication of structured pore scaffolds. For the fabrication of double-pore scaffolds, PVA molds were printed with 40% filler. 10 g of NaCl crystals (Sigma-Aldrich Corporation, St. Louis, MO, USA) (size 300-600 μm) were mixed with 1 ml of ultrapure water from a Milli-Q® water purification system (Millipore Corporation, Billerica, MA, USA) and manually packed into the PVA mold. The salt-filled molds were dried in an oven at 60 °C for 1 h. The small amount of water was used to form interconnections between the salt crystals, as well as between the salt crystals and the PVA filaments. Both PVA molds for structured pore scaffolds and salt-PVA molds for double-pore scaffolds were replicated in polydimethylsiloxane (PDMS) (Sylgard 184 elastomer kit, Dow Corning Corporation, Midland, MI, USA). The PDMS base was thoroughly mixed with the curing agent in a ratio of 10:1 and the mixture was degassed in a vacuum desiccator to remove air bubbles. The PDMS mixture was then poured into the mold, and the container with the mold covered with the PDMS mixture was transferred to the vacuum desiccator for 2-3 h to ensure complete infiltration of PDMS into the mold. The PDMS was cured at 60 °C in an oven for 4-6 h. After curing, the excess PDMS around the molds was removed to expose the PVA or salt-PVA structures. This was done to allow subsequent dissolution of PVA and salt in water. The complete PDMS structures of the molds were immersed in water until the PVA and salt were completely removed (around 6-12 h), releasing the PDMS scaffolds with structured or double-pore architecture. Various physical and mechanical properties of the double-pore scaffolds were compared with control scaffolds with only structured or only random pores, fabricated using previously reported methods. | The scaffolds with double pores showed intermediate mechanical properties compared to scaffolds with structured and random pores. The compression modulus of the structured pore scaffolds was similar to that of our previous study presented with similar type scaffolds. The result of the mechanical test indicates that the mechanical properties of the double pore scaffolds can be easily modified by combining random and structured pores. Since the elastic moduli of human organs and tissues are highly diverse, the stiffness of the fabricated double pore PDMS scaffolds can be adjusted by changing the porosity of the scaffolds or the degree of crosslinking, i.e., the ratio between PDMS base and curing agent [36]. As mentioned earlier, the manufacturing technique presented in this document can be applied to other materials besides PDMS, such as the biomaterial PCL (supplementary material Fig. S5), which provides an additional way to fine-tune the stiffness of the scaffolds according to the needs of in vivo tissues. The double pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. | A method for manufacturing double-pore scaffolds by combining 3D filament printing of a water-soluble sacrificial PVA mold for the primary structured pores and salt leaching to create random pores within the bulk scaffold. The method allows for control of pore geometry and dimensions, as well as the mechanical stiffness of the structured and random pores of the double-pore scaffold. Using PDMS as the scaffold material, the manufacturing process was demonstrated to be fast, inexpensive, and scalable for generating scaffolds of a size relevant to thick tissues. Sacrificial molds printed with PVA can also be used with different biodegradable natural polymers (e.g., gelatin and silk) combined with lyophilization to provide scaffolds with a wide range of mechanical properties. Human hepatoblastoma cells (HepG2) were cultured on different types of scaffolds for 16 days, monitoring their adhesion, distribution, proliferation, viability, metabolic activity, and albumin secretion, and cells were cultured on double-pore scaffolds with cells cultured on scaffolds with only structured or only random pores. The double-pore scaffold with highly interconnected structured pores combined with adjacent random pores was superior to the other two types of scaffolds as it combines a high number of cells, metabolic activity, and albumin secretion with a uniform distribution of live cells throughout the scaffold volume. In conclusion, the described manufacturing technique is fast, inexpensive, scalable, and compatible with different polymers, making it suitable for engineering various organs/tissues on a large scale. | Legal Aspects | It must be bio-compatible. | PDMS is chosen as the material for the final scaffold. | In the United States, the FDA approves the use of dimethicone as safe, as it has been widely used for many years. |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME16 | Presents a cutting-edge study of three-dimensional printing technologies for tissue engineering applications, with special attention to the development of a computer-aided scaffold design system; direct 3D printing of functionally graded scaffolds; modeling of selective laser sintering (SLS) and fused deposition modeling (FDM) processes; indirect additive manufacturing of scaffolds, with both micro and macro features; the development of a bioreactor; and 3D/4D bio-printing. Technological limitations will be examined to highlight the possibility of future improvements in new three-dimensional printing methodologies for tissue engineering. | Literature review | Architecture design of scaffold. We have adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. Using this approach, we can fine-tune the mechanical property, based on the design of the porous structure. We have developed a computer-assisted scaffold design system (CASTS) that can automatically create a highly porous 3D scaffold model with a controlled architecture, and that exactly matches the external surface profile of a native anatomical structure such as bone [10-12]. Natural tissues, such as bone, often have a gradient porous structure, so it is important that the mechanical strength and stiffness between the porous scaffold and the target tissue structure are equal [14]. There are two types of stiffness gradient in bones: radial gradients in long bones, and linear gradients in short and irregular bones. We have achieved the design of radial gradient by arranging the cylindrical unit cells concentrically so that the porosity decreases linearly from the center to the periphery. This linear gradient is produced as a result of varying the strut diameter along the gradient direction. Therefore, we can tailor the stiffness variation for the plaster scaffolds by adjusting the porosity-hardness relationship. The results show that a designed pore size of 250 μm or larger promotes blood vessel growth more than smaller pore sizes [21]. In addition, high porosity does not necessarily lead to increased vascularization, because cell migration and vascularization may be inhibited if there is little interconnectivity between the pores [22]. Recently, researchers have developed a toolbox for evaluating 3D porous scaffolds [23]. This toolbox is based on a modular scaffold design, and allows fine-tuning of scaffold pore size and porosity for vascularization study. *Direct printing. as the success of vertical dimension printing also depends on the adhesion strength between layers. Therefore, when exploring a material for 3D scaffold applications, it is important to consider the available forms of the material in the first stage. Furthermore, to increase the range of 3D printable biomaterials, future development should include the invention of new methods to transform existing biomaterials into suitable forms for 3D printing. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be kept at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be kept at 30% by weight or less to obtain satisfactory scaffold samples with well-defined pore interconnectivity and good structural integrity although the addition of HA initially improved mechanical properties and bioactivity, it compromised material properties during the hydrolytic degradation process [45]. A limitation in the SLS process is the waste of material when building small prototypes such as tissue engineering scaffolds. However, this problem can be overcome by incorporating a compact adaptation system into the SLS partial bed, allowing the adapter to transfer the movement of the SLS partial bed to its own small partial bed [56]. Up to 6.5 times the amount of powder can be saved using this device. A second limitation of scaffolds made with SLS is the low cell retention during cell seeding. One reason is that the materials used for SLS are synthetic and do not favor initial cell attachment. The other reason is that the pores are much larger than the cells due to the issue of SLS resolution, as a result, cells fall through the pores during the seeding process. However, the use of a hybrid 3D scaffold consisting of alternating electrospun nanofibers and 3D printed scaffold layers will prevent cells from falling through them, due to the small pore size of the nanofibers [57, 58]. An alternative solution is to inject cell-loaded collagen hydrogel into the porous structure [59]. There is an unresolved limitation in SLS scaffolds, for example, dust trapping in the inner region of the porous scaffold. It is difficult to manually remove trapped dust, especially for pore sizes below 500 μm. Researchers have explored ultrasonic cleaning with limited success [60]. We obtained such a graph for FDM, based on experimental data from ABS samples [63, 64]. Furthermore, we found feasible porosity and compression stiffness range for our PCL scaffolds designed by the mold system through physical prototype testing [65]. This stiffness range fairly matches the stiffness gradient of cancellous bone in the maxillofacial region, which gradually varies from 35.55 MPa in the molar region to 67.48 MPa in the incisive and canine region [66]. *Indirect printing. unlike direct 3D printing, which produces a scaffold directly from the model material, indirect 3D printing creates a negative mold, usually from a support material, and then casts the desired polymer scaffold out of the mold using a drying method [6, 67, 68]. Collagen scaffolds with internal channel 3D networks can be produced using this approach [69]. Furthermore, freeze-drying was found to be the most suitable drying method in indirect 3D printing, as it induced less shrinkage than critical point drying and accurately reproduced the morphology of the channel design [ Note: The translation has been provided without quotation and double quotation marks at the start and end. | In the second decade after the birth of tissue engineering, 3D printing gradually became a definitive part of this field, due to its control and manufacturing capability. Looking ahead, even once the aforementioned technical challenges are overcome, there will still be a long way to go in transforming academic knowledge into clinical products that benefit society. The current tasks of researchers in this field involve accelerating the standardization and certification of 3D printed medical devices. Prolonged delays in this standardization would further complicate regulatory efforts, especially with three-dimensional bioprinting technologies, which are currently trending and transforming, as the definition of medical device could soon be redefined. Another future trend could emerge in the legal landscape [95], as infringement and protection of intellectual property surrounding 3D printing become more intertwined. Therefore, an early and informed exploration of various legal approaches could be the best preparation for addressing future changes. | Function | Direct/Indirect Printing: Ideally, three-dimensional scaffolds should be highly porous, have well-interconnected pore networks, and have a constant and suitable pore size for cell migration and infiltration [2]. | The researchers proposed the use of 3D printing methods to manufacture customized scaffolds with controlled size and pore structure [4-6]. Fused deposition modeling (FDM), stereolithography, inkjet printing, selective laser sintering (SLS), and color inkjet printing appeared to be the most popular, due to their ability to process plastics. We adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. Using this approach, we can fine-tune the mechanical property, based on the design of the porous structure. We have developed a computer-assisted system for tissue scaffolds (CASTS) that can automatically create a highly porous 3D scaffold model with controlled architecture, and that exactly matches the external surface profile of a native anatomical structure such as bone [10-12]. | In this system, nearly 20 polyhedral shapes are selected to form the basic geometry of a unit cell. The scaffold library and parameters of each unit cell, such as pore size and strut size, can be adjusted, and each polyhedral unit can be automatically repeated in a spatial arrangement and dimensioned to form a block that fits the intended application of the scaffold (Figure 1). Next, an anatomically shaped porous scaffold can be created by a Boolean operation between the scaffold block and the actual model of the tissue surface defect. A detailed derivation of the mathematical formulas of the CASTS system for designing and manufacturing tissue engineering scaffolds can be found in Ref. [13]. |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME16 | Presents a cutting-edge study of three-dimensional printing technologies for tissue engineering applications, with special attention to the development of a computer-aided scaffold design system; direct 3D printing of functionally graded scaffolds; modeling of selective laser sintering (SLS) and fused deposition modeling (FDM) processes; indirect additive manufacturing of scaffolds, with both micro and macro features; the development of a bioreactor; and 3D/4D bio-printing. Technological limitations will be examined to highlight the possibility of future improvements in new three-dimensional printing methodologies for tissue engineering. | Literature review | Architecture design of scaffold. We have adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. Using this approach, we can fine-tune the mechanical property, based on the design of the porous structure. We have developed a computer-assisted scaffold design system (CASTS) that can automatically create a highly porous 3D scaffold model with a controlled architecture, and that exactly matches the external surface profile of a native anatomical structure such as bone [10-12]. Natural tissues, such as bone, often have a gradient porous structure, so it is important that the mechanical strength and stiffness between the porous scaffold and the target tissue structure are equal [14]. There are two types of stiffness gradient in bones: radial gradients in long bones, and linear gradients in short and irregular bones. We have achieved the design of radial gradient by arranging the cylindrical unit cells concentrically so that the porosity decreases linearly from the center to the periphery. This linear gradient is produced as a result of varying the strut diameter along the gradient direction. Therefore, we can tailor the stiffness variation for the plaster scaffolds by adjusting the porosity-hardness relationship. The results show that a designed pore size of 250 μm or larger promotes blood vessel growth more than smaller pore sizes [21]. In addition, high porosity does not necessarily lead to increased vascularization, because cell migration and vascularization may be inhibited if there is little interconnectivity between the pores [22]. Recently, researchers have developed a toolbox for evaluating 3D porous scaffolds [23]. This toolbox is based on a modular scaffold design, and allows fine-tuning of scaffold pore size and porosity for vascularization study. *Direct printing. as the success of vertical dimension printing also depends on the adhesion strength between layers. Therefore, when exploring a material for 3D scaffold applications, it is important to consider the available forms of the material in the first stage. Furthermore, to increase the range of 3D printable biomaterials, future development should include the invention of new methods to transform existing biomaterials into suitable forms for 3D printing. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be kept at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be kept at 30% by weight or less to obtain satisfactory scaffold samples with well-defined pore interconnectivity and good structural integrity although the addition of HA initially improved mechanical properties and bioactivity, it compromised material properties during the hydrolytic degradation process [45]. A limitation in the SLS process is the waste of material when building small prototypes such as tissue engineering scaffolds. However, this problem can be overcome by incorporating a compact adaptation system into the SLS partial bed, allowing the adapter to transfer the movement of the SLS partial bed to its own small partial bed [56]. Up to 6.5 times the amount of powder can be saved using this device. A second limitation of scaffolds made with SLS is the low cell retention during cell seeding. One reason is that the materials used for SLS are synthetic and do not favor initial cell attachment. The other reason is that the pores are much larger than the cells due to the issue of SLS resolution, as a result, cells fall through the pores during the seeding process. However, the use of a hybrid 3D scaffold consisting of alternating electrospun nanofibers and 3D printed scaffold layers will prevent cells from falling through them, due to the small pore size of the nanofibers [57, 58]. An alternative solution is to inject cell-loaded collagen hydrogel into the porous structure [59]. There is an unresolved limitation in SLS scaffolds, for example, dust trapping in the inner region of the porous scaffold. It is difficult to manually remove trapped dust, especially for pore sizes below 500 μm. Researchers have explored ultrasonic cleaning with limited success [60]. We obtained such a graph for FDM, based on experimental data from ABS samples [63, 64]. Furthermore, we found feasible porosity and compression stiffness range for our PCL scaffolds designed by the mold system through physical prototype testing [65]. This stiffness range fairly matches the stiffness gradient of cancellous bone in the maxillofacial region, which gradually varies from 35.55 MPa in the molar region to 67.48 MPa in the incisive and canine region [66]. *Indirect printing. unlike direct 3D printing, which produces a scaffold directly from the model material, indirect 3D printing creates a negative mold, usually from a support material, and then casts the desired polymer scaffold out of the mold using a drying method [6, 67, 68]. Collagen scaffolds with internal channel 3D networks can be produced using this approach [69]. Furthermore, freeze-drying was found to be the most suitable drying method in indirect 3D printing, as it induced less shrinkage than critical point drying and accurately reproduced the morphology of the channel design [ Note: The translation has been provided without quotation and double quotation marks at the start and end. | In the second decade after the birth of tissue engineering, 3D printing gradually became a definitive part of this field, due to its control and manufacturing capability. Looking ahead, even once the aforementioned technical challenges are overcome, there will still be a long way to go in transforming academic knowledge into clinical products that benefit society. The current tasks of researchers in this field involve accelerating the standardization and certification of 3D printed medical devices. Prolonged delays in this standardization would further complicate regulatory efforts, especially with three-dimensional bioprinting technologies, which are currently trending and transforming, as the definition of medical device could soon be redefined. Another future trend could emerge in the legal landscape [95], as infringement and protection of intellectual property surrounding 3D printing become more intertwined. Therefore, an early and informed exploration of various legal approaches could be the best preparation for addressing future changes. | Dimensions/ customization | Direct/indirect printing: Ideally, three-dimensional scaffolds should be highly porous, have well-interconnected pore networks, and have a constant and appropriate pore size for cell migration and infiltration [2]. Natural tissues, such as bone, typically have a gradient porous structure, so it is important that the mechanical strength and stiffness between the porous scaffold and the target tissue structure are equal [14]. There are two types of stiffness gradients in bones: radial gradients in long bones, and linear gradients in short and irregular bones. | Direct printing: we adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. We have achieved the design of the radial gradient. Another method for designing gradient structures is based on the shape function and refinement of the hexagonal mesh [18]. A new method based on the sigmoid function and the radial basis function has recently been developed to determine the growth rate of structures with functional grading, and the resulting models can be exported as STL files and 3D printed [19]. The results show that a designed pore size of 250 μm or larger promotes the growth of blood vessels more than smaller pore sizes [21]. | Direct printing: In this system, nearly 20 polyhedral forms are selected to form the basic geometry of a unit cell. The scaffold library and parameters of each unit cell, such as pore size and strut size, can be adjusted, and each polyhedral unit can be automatically repeated in a spatial arrangement and dimensioned to form a block that fits the intended application of the scaffold (Figure 1). Then, an anatomically shaped porous scaffold can be created by a Boolean operation between the scaffold block and the actual model of the tissue surface defect. A detailed derivation of the mathematical formulas of the CASTS system for designing and manufacturing tissue engineering scaffolds can be found in Ref. [13]. We have achieved the design of radial gradient by arranging the cylindrical unit cells concentrically so that the porosity decreases linearly from the center to the periphery. This linear gradient is achieved by varying the strut diameter along the gradient direction. Therefore, we can tailor the stiffness variation for gypsum scaffolds by adjusting the porosity-hardness relationship. Hexagonal shape and refinement function: In this method, a truncated bone is subdivided and represented using various irregular hexahedral elements, which are then converted into various irregular pore elements based on the shape function. The complete pore model is obtained after a union operation between the irregular pores, and then the resulting bone fold is obtained by performing a difference operation between the contour model and the pore model. With this method, a well-defined pore size distribution can be achieved for gradient bone scaffold design. In addition, high porosity does not necessarily lead to increased vascularization, as cell migration and vascularization may be inhibited if there is little interconnectivity between the pores [22]. |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME16 | Presents a cutting-edge study of three-dimensional printing technologies for tissue engineering applications, with special attention to the development of a computer-aided scaffold design system; direct 3D printing of functionally graded scaffolds; modeling of selective laser sintering (SLS) and fused deposition modeling (FDM) processes; indirect additive manufacturing of scaffolds, with both micro and macro features; the development of a bioreactor; and 3D/4D bio-printing. Technological limitations will be examined to highlight the possibility of future improvements in new three-dimensional printing methodologies for tissue engineering. | Literature review | Architecture design of scaffold. We have adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. Using this approach, we can fine-tune the mechanical property, based on the design of the porous structure. We have developed a computer-assisted scaffold design system (CASTS) that can automatically create a highly porous 3D scaffold model with a controlled architecture, and that exactly matches the external surface profile of a native anatomical structure such as bone [10-12]. Natural tissues, such as bone, often have a gradient porous structure, so it is important that the mechanical strength and stiffness between the porous scaffold and the target tissue structure are equal [14]. There are two types of stiffness gradient in bones: radial gradients in long bones, and linear gradients in short and irregular bones. We have achieved the design of radial gradient by arranging the cylindrical unit cells concentrically so that the porosity decreases linearly from the center to the periphery. This linear gradient is produced as a result of varying the strut diameter along the gradient direction. Therefore, we can tailor the stiffness variation for the plaster scaffolds by adjusting the porosity-hardness relationship. The results show that a designed pore size of 250 μm or larger promotes blood vessel growth more than smaller pore sizes [21]. In addition, high porosity does not necessarily lead to increased vascularization, because cell migration and vascularization may be inhibited if there is little interconnectivity between the pores [22]. Recently, researchers have developed a toolbox for evaluating 3D porous scaffolds [23]. This toolbox is based on a modular scaffold design, and allows fine-tuning of scaffold pore size and porosity for vascularization study. *Direct printing. as the success of vertical dimension printing also depends on the adhesion strength between layers. Therefore, when exploring a material for 3D scaffold applications, it is important to consider the available forms of the material in the first stage. Furthermore, to increase the range of 3D printable biomaterials, future development should include the invention of new methods to transform existing biomaterials into suitable forms for 3D printing. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be kept at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be kept at 30% by weight or less to obtain satisfactory scaffold samples with well-defined pore interconnectivity and good structural integrity although the addition of HA initially improved mechanical properties and bioactivity, it compromised material properties during the hydrolytic degradation process [45]. A limitation in the SLS process is the waste of material when building small prototypes such as tissue engineering scaffolds. However, this problem can be overcome by incorporating a compact adaptation system into the SLS partial bed, allowing the adapter to transfer the movement of the SLS partial bed to its own small partial bed [56]. Up to 6.5 times the amount of powder can be saved using this device. A second limitation of scaffolds made with SLS is the low cell retention during cell seeding. One reason is that the materials used for SLS are synthetic and do not favor initial cell attachment. The other reason is that the pores are much larger than the cells due to the issue of SLS resolution, as a result, cells fall through the pores during the seeding process. However, the use of a hybrid 3D scaffold consisting of alternating electrospun nanofibers and 3D printed scaffold layers will prevent cells from falling through them, due to the small pore size of the nanofibers [57, 58]. An alternative solution is to inject cell-loaded collagen hydrogel into the porous structure [59]. There is an unresolved limitation in SLS scaffolds, for example, dust trapping in the inner region of the porous scaffold. It is difficult to manually remove trapped dust, especially for pore sizes below 500 μm. Researchers have explored ultrasonic cleaning with limited success [60]. We obtained such a graph for FDM, based on experimental data from ABS samples [63, 64]. Furthermore, we found feasible porosity and compression stiffness range for our PCL scaffolds designed by the mold system through physical prototype testing [65]. This stiffness range fairly matches the stiffness gradient of cancellous bone in the maxillofacial region, which gradually varies from 35.55 MPa in the molar region to 67.48 MPa in the incisive and canine region [66]. *Indirect printing. unlike direct 3D printing, which produces a scaffold directly from the model material, indirect 3D printing creates a negative mold, usually from a support material, and then casts the desired polymer scaffold out of the mold using a drying method [6, 67, 68]. Collagen scaffolds with internal channel 3D networks can be produced using this approach [69]. Furthermore, freeze-drying was found to be the most suitable drying method in indirect 3D printing, as it induced less shrinkage than critical point drying and accurately reproduced the morphology of the channel design [ Note: The translation has been provided without quotation and double quotation marks at the start and end. | In the second decade after the birth of tissue engineering, 3D printing gradually became a definitive part of this field, due to its control and manufacturing capability. Looking ahead, even once the aforementioned technical challenges are overcome, there will still be a long way to go in transforming academic knowledge into clinical products that benefit society. The current tasks of researchers in this field involve accelerating the standardization and certification of 3D printed medical devices. Prolonged delays in this standardization would further complicate regulatory efforts, especially with three-dimensional bioprinting technologies, which are currently trending and transforming, as the definition of medical device could soon be redefined. Another future trend could emerge in the legal landscape [95], as infringement and protection of intellectual property surrounding 3D printing become more intertwined. Therefore, an early and informed exploration of various legal approaches could be the best preparation for addressing future changes. | Dimensions/ customization | - | *Indirect printing: Unlike direct 3D printing, which produces a scaffold directly from the model material, indirect 3D printing creates a negative mold, usually from a support material, and then casts the desired polymer scaffold out of the mold using a drying method [6, 67, 68]. | Indirect printing: Collagen scaffolds with 3D networks of internal channels can be produced using this approach [69]. Furthermore, it was found that freeze-drying was the most suitable drying method in indirect 3D printing, as it induced less shrinkage than critical point drying and accurately reproduced the morphology of the channel design [69]. |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME16 | Presents a cutting-edge study of three-dimensional printing technologies for tissue engineering applications, with special attention to the development of a computer-aided scaffold design system; direct 3D printing of functionally graded scaffolds; modeling of selective laser sintering (SLS) and fused deposition modeling (FDM) processes; indirect additive manufacturing of scaffolds, with both micro and macro features; the development of a bioreactor; and 3D/4D bio-printing. Technological limitations will be examined to highlight the possibility of future improvements in new three-dimensional printing methodologies for tissue engineering. | Literature review | Architecture design of scaffold. We have adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. Using this approach, we can fine-tune the mechanical property, based on the design of the porous structure. We have developed a computer-assisted scaffold design system (CASTS) that can automatically create a highly porous 3D scaffold model with a controlled architecture, and that exactly matches the external surface profile of a native anatomical structure such as bone [10-12]. Natural tissues, such as bone, often have a gradient porous structure, so it is important that the mechanical strength and stiffness between the porous scaffold and the target tissue structure are equal [14]. There are two types of stiffness gradient in bones: radial gradients in long bones, and linear gradients in short and irregular bones. We have achieved the design of radial gradient by arranging the cylindrical unit cells concentrically so that the porosity decreases linearly from the center to the periphery. This linear gradient is produced as a result of varying the strut diameter along the gradient direction. Therefore, we can tailor the stiffness variation for the plaster scaffolds by adjusting the porosity-hardness relationship. The results show that a designed pore size of 250 μm or larger promotes blood vessel growth more than smaller pore sizes [21]. In addition, high porosity does not necessarily lead to increased vascularization, because cell migration and vascularization may be inhibited if there is little interconnectivity between the pores [22]. Recently, researchers have developed a toolbox for evaluating 3D porous scaffolds [23]. This toolbox is based on a modular scaffold design, and allows fine-tuning of scaffold pore size and porosity for vascularization study. *Direct printing. as the success of vertical dimension printing also depends on the adhesion strength between layers. Therefore, when exploring a material for 3D scaffold applications, it is important to consider the available forms of the material in the first stage. Furthermore, to increase the range of 3D printable biomaterials, future development should include the invention of new methods to transform existing biomaterials into suitable forms for 3D printing. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be kept at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be kept at 30% by weight or less to obtain satisfactory scaffold samples with well-defined pore interconnectivity and good structural integrity although the addition of HA initially improved mechanical properties and bioactivity, it compromised material properties during the hydrolytic degradation process [45]. A limitation in the SLS process is the waste of material when building small prototypes such as tissue engineering scaffolds. However, this problem can be overcome by incorporating a compact adaptation system into the SLS partial bed, allowing the adapter to transfer the movement of the SLS partial bed to its own small partial bed [56]. Up to 6.5 times the amount of powder can be saved using this device. A second limitation of scaffolds made with SLS is the low cell retention during cell seeding. One reason is that the materials used for SLS are synthetic and do not favor initial cell attachment. The other reason is that the pores are much larger than the cells due to the issue of SLS resolution, as a result, cells fall through the pores during the seeding process. However, the use of a hybrid 3D scaffold consisting of alternating electrospun nanofibers and 3D printed scaffold layers will prevent cells from falling through them, due to the small pore size of the nanofibers [57, 58]. An alternative solution is to inject cell-loaded collagen hydrogel into the porous structure [59]. There is an unresolved limitation in SLS scaffolds, for example, dust trapping in the inner region of the porous scaffold. It is difficult to manually remove trapped dust, especially for pore sizes below 500 μm. Researchers have explored ultrasonic cleaning with limited success [60]. We obtained such a graph for FDM, based on experimental data from ABS samples [63, 64]. Furthermore, we found feasible porosity and compression stiffness range for our PCL scaffolds designed by the mold system through physical prototype testing [65]. This stiffness range fairly matches the stiffness gradient of cancellous bone in the maxillofacial region, which gradually varies from 35.55 MPa in the molar region to 67.48 MPa in the incisive and canine region [66]. *Indirect printing. unlike direct 3D printing, which produces a scaffold directly from the model material, indirect 3D printing creates a negative mold, usually from a support material, and then casts the desired polymer scaffold out of the mold using a drying method [6, 67, 68]. Collagen scaffolds with internal channel 3D networks can be produced using this approach [69]. Furthermore, freeze-drying was found to be the most suitable drying method in indirect 3D printing, as it induced less shrinkage than critical point drying and accurately reproduced the morphology of the channel design [ Note: The translation has been provided without quotation and double quotation marks at the start and end. | In the second decade after the birth of tissue engineering, 3D printing gradually became a definitive part of this field, due to its control and manufacturing capability. Looking ahead, even once the aforementioned technical challenges are overcome, there will still be a long way to go in transforming academic knowledge into clinical products that benefit society. The current tasks of researchers in this field involve accelerating the standardization and certification of 3D printed medical devices. Prolonged delays in this standardization would further complicate regulatory efforts, especially with three-dimensional bioprinting technologies, which are currently trending and transforming, as the definition of medical device could soon be redefined. Another future trend could emerge in the legal landscape [95], as infringement and protection of intellectual property surrounding 3D printing become more intertwined. Therefore, an early and informed exploration of various legal approaches could be the best preparation for addressing future changes. | Strength | Direct/indirect printing: It must have resistance of similar materials where it will be implanted. | We obtained a graph of this type for FDM, based on experimental data from ABS samples [63, 64]. In addition, we found feasible porosity and a range of compression stiffness for our PCL scaffolds designed by the mold system through physical prototype testing [65]. This range of stiffness quite matches the stiffness gradient of trabecular bone in the maxillofacial region, which gradually varies from 35.55 MPa in the molar region to 67.48 MPa in the incisive and canine region [66]. | Experimental method |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME16 | Presents a cutting-edge study of three-dimensional printing technologies for tissue engineering applications, with special attention to the development of a computer-aided scaffold design system; direct 3D printing of functionally graded scaffolds; modeling of selective laser sintering (SLS) and fused deposition modeling (FDM) processes; indirect additive manufacturing of scaffolds, with both micro and macro features; the development of a bioreactor; and 3D/4D bio-printing. Technological limitations will be examined to highlight the possibility of future improvements in new three-dimensional printing methodologies for tissue engineering. | Literature review | Architecture design of scaffold. We have adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. Using this approach, we can fine-tune the mechanical property, based on the design of the porous structure. We have developed a computer-assisted scaffold design system (CASTS) that can automatically create a highly porous 3D scaffold model with a controlled architecture, and that exactly matches the external surface profile of a native anatomical structure such as bone [10-12]. Natural tissues, such as bone, often have a gradient porous structure, so it is important that the mechanical strength and stiffness between the porous scaffold and the target tissue structure are equal [14]. There are two types of stiffness gradient in bones: radial gradients in long bones, and linear gradients in short and irregular bones. We have achieved the design of radial gradient by arranging the cylindrical unit cells concentrically so that the porosity decreases linearly from the center to the periphery. This linear gradient is produced as a result of varying the strut diameter along the gradient direction. Therefore, we can tailor the stiffness variation for the plaster scaffolds by adjusting the porosity-hardness relationship. The results show that a designed pore size of 250 μm or larger promotes blood vessel growth more than smaller pore sizes [21]. In addition, high porosity does not necessarily lead to increased vascularization, because cell migration and vascularization may be inhibited if there is little interconnectivity between the pores [22]. Recently, researchers have developed a toolbox for evaluating 3D porous scaffolds [23]. This toolbox is based on a modular scaffold design, and allows fine-tuning of scaffold pore size and porosity for vascularization study. *Direct printing. as the success of vertical dimension printing also depends on the adhesion strength between layers. Therefore, when exploring a material for 3D scaffold applications, it is important to consider the available forms of the material in the first stage. Furthermore, to increase the range of 3D printable biomaterials, future development should include the invention of new methods to transform existing biomaterials into suitable forms for 3D printing. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be kept at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be kept at 30% by weight or less to obtain satisfactory scaffold samples with well-defined pore interconnectivity and good structural integrity although the addition of HA initially improved mechanical properties and bioactivity, it compromised material properties during the hydrolytic degradation process [45]. A limitation in the SLS process is the waste of material when building small prototypes such as tissue engineering scaffolds. However, this problem can be overcome by incorporating a compact adaptation system into the SLS partial bed, allowing the adapter to transfer the movement of the SLS partial bed to its own small partial bed [56]. Up to 6.5 times the amount of powder can be saved using this device. A second limitation of scaffolds made with SLS is the low cell retention during cell seeding. One reason is that the materials used for SLS are synthetic and do not favor initial cell attachment. The other reason is that the pores are much larger than the cells due to the issue of SLS resolution, as a result, cells fall through the pores during the seeding process. However, the use of a hybrid 3D scaffold consisting of alternating electrospun nanofibers and 3D printed scaffold layers will prevent cells from falling through them, due to the small pore size of the nanofibers [57, 58]. An alternative solution is to inject cell-loaded collagen hydrogel into the porous structure [59]. There is an unresolved limitation in SLS scaffolds, for example, dust trapping in the inner region of the porous scaffold. It is difficult to manually remove trapped dust, especially for pore sizes below 500 μm. Researchers have explored ultrasonic cleaning with limited success [60]. We obtained such a graph for FDM, based on experimental data from ABS samples [63, 64]. Furthermore, we found feasible porosity and compression stiffness range for our PCL scaffolds designed by the mold system through physical prototype testing [65]. This stiffness range fairly matches the stiffness gradient of cancellous bone in the maxillofacial region, which gradually varies from 35.55 MPa in the molar region to 67.48 MPa in the incisive and canine region [66]. *Indirect printing. unlike direct 3D printing, which produces a scaffold directly from the model material, indirect 3D printing creates a negative mold, usually from a support material, and then casts the desired polymer scaffold out of the mold using a drying method [6, 67, 68]. Collagen scaffolds with internal channel 3D networks can be produced using this approach [69]. Furthermore, freeze-drying was found to be the most suitable drying method in indirect 3D printing, as it induced less shrinkage than critical point drying and accurately reproduced the morphology of the channel design [ Note: The translation has been provided without quotation and double quotation marks at the start and end. | In the second decade after the birth of tissue engineering, 3D printing gradually became a definitive part of this field, due to its control and manufacturing capability. Looking ahead, even once the aforementioned technical challenges are overcome, there will still be a long way to go in transforming academic knowledge into clinical products that benefit society. The current tasks of researchers in this field involve accelerating the standardization and certification of 3D printed medical devices. Prolonged delays in this standardization would further complicate regulatory efforts, especially with three-dimensional bioprinting technologies, which are currently trending and transforming, as the definition of medical device could soon be redefined. Another future trend could emerge in the legal landscape [95], as infringement and protection of intellectual property surrounding 3D printing become more intertwined. Therefore, an early and informed exploration of various legal approaches could be the best preparation for addressing future changes. | Material | Direct/indirect printing: Must be biocompatible. | For the SLS, namely, the bed temperature, laser power, and scanning speed. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be maintained at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be maintained at 30% by weight or less to obtain satisfactory scaffold samples with a well-defined pore interconnectivity and good structural integrity. PCL is a representative biomaterial for the FDM process. | |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME16 | Presents a cutting-edge study of three-dimensional printing technologies for tissue engineering applications, with special attention to the development of a computer-aided scaffold design system; direct 3D printing of functionally graded scaffolds; modeling of selective laser sintering (SLS) and fused deposition modeling (FDM) processes; indirect additive manufacturing of scaffolds, with both micro and macro features; the development of a bioreactor; and 3D/4D bio-printing. Technological limitations will be examined to highlight the possibility of future improvements in new three-dimensional printing methodologies for tissue engineering. | Literature review | Architecture design of scaffold. We have adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. Using this approach, we can fine-tune the mechanical property, based on the design of the porous structure. We have developed a computer-assisted scaffold design system (CASTS) that can automatically create a highly porous 3D scaffold model with a controlled architecture, and that exactly matches the external surface profile of a native anatomical structure such as bone [10-12]. Natural tissues, such as bone, often have a gradient porous structure, so it is important that the mechanical strength and stiffness between the porous scaffold and the target tissue structure are equal [14]. There are two types of stiffness gradient in bones: radial gradients in long bones, and linear gradients in short and irregular bones. We have achieved the design of radial gradient by arranging the cylindrical unit cells concentrically so that the porosity decreases linearly from the center to the periphery. This linear gradient is produced as a result of varying the strut diameter along the gradient direction. Therefore, we can tailor the stiffness variation for the plaster scaffolds by adjusting the porosity-hardness relationship. The results show that a designed pore size of 250 μm or larger promotes blood vessel growth more than smaller pore sizes [21]. In addition, high porosity does not necessarily lead to increased vascularization, because cell migration and vascularization may be inhibited if there is little interconnectivity between the pores [22]. Recently, researchers have developed a toolbox for evaluating 3D porous scaffolds [23]. This toolbox is based on a modular scaffold design, and allows fine-tuning of scaffold pore size and porosity for vascularization study. *Direct printing. as the success of vertical dimension printing also depends on the adhesion strength between layers. Therefore, when exploring a material for 3D scaffold applications, it is important to consider the available forms of the material in the first stage. Furthermore, to increase the range of 3D printable biomaterials, future development should include the invention of new methods to transform existing biomaterials into suitable forms for 3D printing. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be kept at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be kept at 30% by weight or less to obtain satisfactory scaffold samples with well-defined pore interconnectivity and good structural integrity although the addition of HA initially improved mechanical properties and bioactivity, it compromised material properties during the hydrolytic degradation process [45]. A limitation in the SLS process is the waste of material when building small prototypes such as tissue engineering scaffolds. However, this problem can be overcome by incorporating a compact adaptation system into the SLS partial bed, allowing the adapter to transfer the movement of the SLS partial bed to its own small partial bed [56]. Up to 6.5 times the amount of powder can be saved using this device. A second limitation of scaffolds made with SLS is the low cell retention during cell seeding. One reason is that the materials used for SLS are synthetic and do not favor initial cell attachment. The other reason is that the pores are much larger than the cells due to the issue of SLS resolution, as a result, cells fall through the pores during the seeding process. However, the use of a hybrid 3D scaffold consisting of alternating electrospun nanofibers and 3D printed scaffold layers will prevent cells from falling through them, due to the small pore size of the nanofibers [57, 58]. An alternative solution is to inject cell-loaded collagen hydrogel into the porous structure [59]. There is an unresolved limitation in SLS scaffolds, for example, dust trapping in the inner region of the porous scaffold. It is difficult to manually remove trapped dust, especially for pore sizes below 500 μm. Researchers have explored ultrasonic cleaning with limited success [60]. We obtained such a graph for FDM, based on experimental data from ABS samples [63, 64]. Furthermore, we found feasible porosity and compression stiffness range for our PCL scaffolds designed by the mold system through physical prototype testing [65]. This stiffness range fairly matches the stiffness gradient of cancellous bone in the maxillofacial region, which gradually varies from 35.55 MPa in the molar region to 67.48 MPa in the incisive and canine region [66]. *Indirect printing. unlike direct 3D printing, which produces a scaffold directly from the model material, indirect 3D printing creates a negative mold, usually from a support material, and then casts the desired polymer scaffold out of the mold using a drying method [6, 67, 68]. Collagen scaffolds with internal channel 3D networks can be produced using this approach [69]. Furthermore, freeze-drying was found to be the most suitable drying method in indirect 3D printing, as it induced less shrinkage than critical point drying and accurately reproduced the morphology of the channel design [ Note: The translation has been provided without quotation and double quotation marks at the start and end. | In the second decade after the birth of tissue engineering, 3D printing gradually became a definitive part of this field, due to its control and manufacturing capability. Looking ahead, even once the aforementioned technical challenges are overcome, there will still be a long way to go in transforming academic knowledge into clinical products that benefit society. The current tasks of researchers in this field involve accelerating the standardization and certification of 3D printed medical devices. Prolonged delays in this standardization would further complicate regulatory efforts, especially with three-dimensional bioprinting technologies, which are currently trending and transforming, as the definition of medical device could soon be redefined. Another future trend could emerge in the legal landscape [95], as infringement and protection of intellectual property surrounding 3D printing become more intertwined. Therefore, an early and informed exploration of various legal approaches could be the best preparation for addressing future changes. | Manufacturing and Assembly | Direct/indirect printing: The results show that a designed pore size of 250 μm or larger promotes the growth of blood vessels more than smaller pore sizes. | FDM/SLS/ translated into ingles is FDM/SLS/. | - |
| GME7 | Tissue engineering | Scaffolding for fabric. | ME16 | Presents a cutting-edge study of three-dimensional printing technologies for tissue engineering applications, with special attention to the development of a computer-aided scaffold design system; direct 3D printing of functionally graded scaffolds; modeling of selective laser sintering (SLS) and fused deposition modeling (FDM) processes; indirect additive manufacturing of scaffolds, with both micro and macro features; the development of a bioreactor; and 3D/4D bio-printing. Technological limitations will be examined to highlight the possibility of future improvements in new three-dimensional printing methodologies for tissue engineering. | Literature review | Architecture design of scaffold. We have adopted a bottom-up approach when constructing a 3D scaffold; that is, first making unit cells, and then assembling them into a 3D scaffold. Using this approach, we can fine-tune the mechanical property, based on the design of the porous structure. We have developed a computer-assisted scaffold design system (CASTS) that can automatically create a highly porous 3D scaffold model with a controlled architecture, and that exactly matches the external surface profile of a native anatomical structure such as bone [10-12]. Natural tissues, such as bone, often have a gradient porous structure, so it is important that the mechanical strength and stiffness between the porous scaffold and the target tissue structure are equal [14]. There are two types of stiffness gradient in bones: radial gradients in long bones, and linear gradients in short and irregular bones. We have achieved the design of radial gradient by arranging the cylindrical unit cells concentrically so that the porosity decreases linearly from the center to the periphery. This linear gradient is produced as a result of varying the strut diameter along the gradient direction. Therefore, we can tailor the stiffness variation for the plaster scaffolds by adjusting the porosity-hardness relationship. The results show that a designed pore size of 250 μm or larger promotes blood vessel growth more than smaller pore sizes [21]. In addition, high porosity does not necessarily lead to increased vascularization, because cell migration and vascularization may be inhibited if there is little interconnectivity between the pores [22]. Recently, researchers have developed a toolbox for evaluating 3D porous scaffolds [23]. This toolbox is based on a modular scaffold design, and allows fine-tuning of scaffold pore size and porosity for vascularization study. *Direct printing. as the success of vertical dimension printing also depends on the adhesion strength between layers. Therefore, when exploring a material for 3D scaffold applications, it is important to consider the available forms of the material in the first stage. Furthermore, to increase the range of 3D printable biomaterials, future development should include the invention of new methods to transform existing biomaterials into suitable forms for 3D printing. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be kept at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be kept at 30% by weight or less to obtain satisfactory scaffold samples with well-defined pore interconnectivity and good structural integrity although the addition of HA initially improved mechanical properties and bioactivity, it compromised material properties during the hydrolytic degradation process [45]. A limitation in the SLS process is the waste of material when building small prototypes such as tissue engineering scaffolds. However, this problem can be overcome by incorporating a compact adaptation system into the SLS partial bed, allowing the adapter to transfer the movement of the SLS partial bed to its own small partial bed [56]. Up to 6.5 times the amount of powder can be saved using this device. A second limitation of scaffolds made with SLS is the low cell retention during cell seeding. One reason is that the materials used for SLS are synthetic and do not favor initial cell attachment. The other reason is that the pores are much larger than the cells due to the issue of SLS resolution, as a result, cells fall through the pores during the seeding process. However, the use of a hybrid 3D scaffold consisting of alternating electrospun nanofibers and 3D printed scaffold layers will prevent cells from falling through them, due to the small pore size of the nanofibers [57, 58]. An alternative solution is to inject cell-loaded collagen hydrogel into the porous structure [59]. There is an unresolved limitation in SLS scaffolds, for example, dust trapping in the inner region of the porous scaffold. It is difficult to manually remove trapped dust, especially for pore sizes below 500 μm. Researchers have explored ultrasonic cleaning with limited success [60]. We obtained such a graph for FDM, based on experimental data from ABS samples [63, 64]. Furthermore, we found feasible porosity and compression stiffness range for our PCL scaffolds designed by the mold system through physical prototype testing [65]. This stiffness range fairly matches the stiffness gradient of cancellous bone in the maxillofacial region, which gradually varies from 35.55 MPa in the molar region to 67.48 MPa in the incisive and canine region [66]. *Indirect printing. unlike direct 3D printing, which produces a scaffold directly from the model material, indirect 3D printing creates a negative mold, usually from a support material, and then casts the desired polymer scaffold out of the mold using a drying method [6, 67, 68]. Collagen scaffolds with internal channel 3D networks can be produced using this approach [69]. Furthermore, freeze-drying was found to be the most suitable drying method in indirect 3D printing, as it induced less shrinkage than critical point drying and accurately reproduced the morphology of the channel design [ Note: The translation has been provided without quotation and double quotation marks at the start and end. | In the second decade after the birth of tissue engineering, 3D printing gradually became a definitive part of this field, due to its control and manufacturing capability. Looking ahead, even once the aforementioned technical challenges are overcome, there will still be a long way to go in transforming academic knowledge into clinical products that benefit society. The current tasks of researchers in this field involve accelerating the standardization and certification of 3D printed medical devices. Prolonged delays in this standardization would further complicate regulatory efforts, especially with three-dimensional bioprinting technologies, which are currently trending and transforming, as the definition of medical device could soon be redefined. Another future trend could emerge in the legal landscape [95], as infringement and protection of intellectual property surrounding 3D printing become more intertwined. Therefore, an early and informed exploration of various legal approaches could be the best preparation for addressing future changes. | Security/Legal aspects | Direct/indirect printing: Must be biocompatible. | For the SLS, namely, the bed temperature, laser power, and scanning speed. In particular, in polyetherketone/hydroxyapatite (PEEK/HA), the results show that HA should be maintained at 40% by weight or less to ensure structural integrity. In polyvinyl alcohol/hydroxyapatite (PVA/HA) and polycaprolactone/hydroxyapatite (PCL/HA) systems, HA should be maintained at 30% by weight or less to obtain satisfactory scaffold samples with a well-defined pore interconnectivity and good structural integrity. PCL is a representative biomaterial for the FDM process. | - |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with the holes positioned on the side and another with the holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | Function | Drug container for sustained release, zero release formulations, at a specific rate for treatment. | 3D printing has the potential to manufacture zero-release formulations with complex geometries. 3D printed scaffolds were designed with different hole positions (side and top/bottom), hole numbers (4, 8, and 12), and hole diameters (1, 1.5, and 2 mm). Dissolution tests and high-performance liquid chromatography analysis were performed. | The fluctuations in drug serum concentrations are reduced, thus reducing the side effects. The usual dose of carbamazepine for epilepsy in adults is 800-1600 mg/day, which corresponds to a release rate of 0.56-1.11 mg/min. The maximum rate obtained in this study was 0.0126 mg/min, which would not be sufficient to reach the therapeutic dose. However, this study has shown that an increase in the diameter of the scaffold holes can lead to an increase in the drug release rate. Therefore, future studies can explore the optimal size of the holes that will produce the desired drug dissolution rate. The low drug release rate can also be avoided by increasing the amount of carbamazepine in the scaffold. |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with the holes positioned on the side and another with the holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | Dimensions | The container must have a volume according to the dose, it must have holes that allow the release of the medication. | Two scaffold designs were 3D printed with ABS, with the holes positioned on the side and the other with the holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's printing resolution, the scaffold cap was designed with a diameter of 14 mm to fit exactly into the scaffold body. The thickness of the cap was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cap and the scaffold body to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Two hundred thousand milligrams of rhodamine B were used to pack into each scaffold. The carbamazepine scaffolds had a diameter of 15 mm, a height of 6 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. The cap was designed with a diameter of 12 mm to fit exactly into the scaffold body. In order to determine the optimal scaffold geometry, each designed scaffold had a different permutation of hole diameter (1, 1.5, and 2 mm) and number of holes (4, 8, and 12 holes). | Experimental method of trial and error. |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with holes positioned on the side and another with holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | Material | It should be bio-compatible. | ABS, PLA, polyvinyl alcohol, ethylene vinyl acetate, polycaprolactone | To the best of our knowledge, there are no studies that have focused on the use of ABS in pharmaceutical applications. Recent studies have shown that ABS scaffolds are biocompatible for cartilage regeneration and nucleus pulposus tissue regeneration, as well as ear-shaped scaffolds for skin cell culture. Although ABS is not bioresorbable, it can potentially be used in pharmaceutical applications due to its biocompatibility and strong chemical resistance, allowing it to safely exit the body like most foreign bodies. However, further studies are needed to address current concerns about the possible leaching of acrylonitrile monomers, which can be carcinogenic. In addition to ABS, various polymers have also been used in the pharmaceutical industry for 3D printing of drug delivery systems using the FDM technique. An example is the use of polylactic acid as a drug eluting implant for slow and partial release of nitrofurantoin and hydroxyapatite antibiotics. Polyvinyl alcohol has also been used as a drug carrier for investigating the effects of geometry on drug release from 3D printed tablets. In fact, poly(vinyl alcohol) has been used as a drug carrier for modified release tablets of aminosalicylate, extended release tablets of prednisolone, and modified release tablets of budesonide. Furthermore, ethylene vinyl acetate has been used as a novel drug carrier for a 3D printed T-shaped intrauterine drug delivery system for indomethacin. In a similar study, polycaprolactone has also been used to deliver indomethacin in a controlled manner in a 3D printed prototype of an intrauterine T-shaped implantable system based on polycaprolactone. Considering the wide variety of polymers that can potentially be used for drug delivery systems, we encourage future studies to also explore these polymers for drug scaffold designs, such as carbamazepine. |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with the holes positioned on the side and another with the holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | Manufacturing and Assembly | The technique used should be cost-effective and widely available. | FDM | FDM is often the cheapest of all 3DP techniques and therefore more affordable for the general public. In addition, the materials used are usually inert polymers that offer great mechanical strength. For 3DP to achieve large-scale therapy customization, the technique used would have to be cost-effective and widely available. FDM seems to be a suitable option for this purpose. Therefore, this study tests the hypothesis of whether 3DP (FDM) can be used to make a zero-order drug release dosage form for carbamazepine. |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with holes positioned on the side and another with holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | assembly | It should facilitate manual packing of the dose and ensure that dissolution is only done through the small holes designed for that purpose. | Radamina B: the cover of the scaffold was designed with a diameter of 14 mm to fit exactly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that the release of the drug in the medium only occurred through the holes that were designed. | |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with holes positioned on the side and another with holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | assembly | It should facilitate manual packing of the dose and ensure that dissolution is only done through the small holes designed for that purpose. | Carbamazepine: The cap was designed with a diameter of 12 mm to fit exactly into the scaffold body. In order to determine the optimal scaffold geometry, each designed scaffold had a different permutation of hole diameter (1, 1.5, and 2 mm) and number of holes (4, 8, and 12 holes). | - |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with the holes positioned on the side and another with the holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | Costs and deadlines | It must be economical | Selected FDM | The technique used should be cost-effective and widely available. |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with holes positioned on the side and another with holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | Security | It must be bio-compatible. | ABS for FDM | Its strong chemical resistance allows it to safely exit the body, as is the case with most foreign bodies. However, further studies need to be conducted to address current concerns about the potential leaching of acrylonitrile monomers, which can be carcinogenic. |
| GME8 | Medicines | Controlled/ sustained release medications | ME5 | This study tests the hypothesis of whether 3DP (FDM) can be used to create a zero-order drug release dosage form for carbamazepine. The aim of this study is to design and investigate 3D printed scaffolds with different pore parameters to determine the optimal parameters that can release carbamazepine in a zero-order manner. | The Rhodamine B (drug substitute) and sodium dodecyl sulfate (SDS, 99%; used as a dissolution medium) were obtained from Alfa Aesar (Massachusetts, MA). The phosphate-buffered saline (PBS, 10, Ultra Pure Grade; used as a dissolution medium) was obtained from Vivantis (Selangor Darul Ehsan, Malaysia). The analytical grade carbamazepine was obtained from Sigma-Aldrich (St. Louis, MO). The Tegretol 200 tablets (carbamazepine) were obtained from Novartis (Basel, Switzerland). The scaffolds were designed to have a cup-shaped body, with a cover that would seal it after packing the drug inside (Fig. 1). The three-dimensional model of the scaffolds was created using AutoCad 2015 (Autodesk, San Francisco, CA). Edges were constructed on the scaffolds to secure the cover to the body of the scaffold. The purpose of this design was to allow the scaffold to be sealed after packing the Rhodamine B into the body of the scaffold, so that the only way to release the drug into the environment was through the holes, thus ensuring a constant surface for interaction. The 3D models were printed using a Da Vinci 1.0 3D printer and XYZware software (XYZprinting, CA). The printing filament used by the 3D printer was a 1.75 mm diameter acrylonitrile butadiene styrene (ABS) filament. The printing settings used were the highest density (90%), the finest layer height (0.1 mm) for the highest resolution, and the standard printing speed defined by the software. Two scaffold designs were 3D printed with ABS, one with holes positioned on the side and another with holes positioned on the top/bottom. Both designs had a diameter of 17 mm, a wall thickness of 1 mm, and a base thickness of 1.5 mm. To account for the 3D printer's resolution, the scaffold cover was designed with a diameter of 14 mm to fit snugly into the body of the scaffold. The thickness of the cover was 1.5 mm. A thin layer of paraffin film was used to cover the small gaps between the cover and the body of the scaffold to ensure that drug release into the medium only occurred through the designed holes. All lengths were measured from the 3D model of the scaffolds. 3D models of the scaffolds were constructed with different positions (side vs. top and bottom), hole diameters (1.5 and 2 mm), and number of holes (4, 5, 8, 12). Dissolution tests and high-performance liquid chromatography analysis were performed. | The scaffolds with holes on the side exhibited a zero-order dissolution kinetics representative of a sustained-release tablet. The results showed that scaffolds with 12 holes and 1 mm diameter produced the most linear profiles. The scaffold design was able to release drugs reproducibly at a constant rate after 5 minutes. It was observed that all scaffolds had drug and excipient residues after 8 hours of testing, so we extrapolated that the disintegration and dissolution process lasted at least 8 hours. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. There was no clear association between the number of scaffold holes and the release rate of carbamazepine, except in the group of scaffolds with a hole diameter of 2 mm. Using rhodamine B as a drug substitute, our study demonstrated that placing the holes on the side resulted in more linear release profiles than holes on the top and bottom of the 3D-printed scaffolds. Of the 3 scaffold configurations (holes on the top, holes on the side, and holes on both the top and bottom), scaffolds with side holes produced a considerably better linear release profile than the other 2 configurations, with an R2 of 0.917 compared to 0.776 and 0.808. Other changes in the scaffolds with side holes, in terms of the number and diameter of the holes, also demonstrated good linearity, except in the scaffold with 8 holes of 1.5 mm diameter and the scaffold with 12 holes of 2 mm diameter. In all scaffolds using carbamazepine, good linear release profiles of carbamazepine were observed, with an R2 value of at least 0.91. Overall, increasing the hole diameters increased the release rate of carbamazepine from the scaffolds while maintaining the linearity of the drug release profile. | This study has shown that 3DP is a useful technique for designing scaffolds that have linear release profiles after 5 minutes, with carbamazepine as our drug of choice. This study has also demonstrated that the diameters of the holes in the printed scaffolds have a positive relationship with the release rate of carbamazepine, but not with the number of holes. The scaffold design described in this study forms a basis that can be further optimized for zero-order release dosage form, to reduce CNS dose-dependent side effects of carbamazepine. The ultimate goal of this research is to benefit epilepsy patients by achieving the therapeutic doses necessary for seizure control through minimizing DEA side effects, and improving patient compliance through a dosage that provides a zero-order drug release profile. | Legal Aspects | It must be bio-compatible. | ABS for FDM | Its strong chemical resistance allows it to safely exit the body, as is the case with most foreign bodies. However, further studies need to be conducted to address current concerns about the potential leaching of acrylonitrile monomers, which can be carcinogenic. |
It is clarified that the references column contains the coded references, but the database also contains additional columns that specify the original reference’s objectives, methods, results, and conclusions.
Consult the database of bibliographic references below [3]-[4].
References
| year (in Data base) | References codified | Title | author | year | Journal or source | use | case | subcase | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 1 | In the search of design for rapid manufacturing strategies to solve functional and geometrical issues for small series production | Javier Munguia, Carles Riba and Joaquim Lloveras | 2007 | Proceedings of ICED 2007, the 16th International Conference on Engineering Design DS 42 | State of the art methodologies | design methodology | ensemble/DFRM | ||||
| 2017 | 2 | An integrated parameterized tool for designing a customized tracheal stent | Evila L. Melgoza, Lídia Serenó, Antoni Rosell, Joaquim Ciurana | 2012 | Computer-Aided Design 44 (2012) 1173–1181. | State of the art methodologies | design methodology | customized/medicine/innovation | ||||
| 2017 | 3 | A novel methodology of design for Additive Manufacturing applied to Additive Laser Manufacturing process | RemiPonche, Olivier Kerbrat, Pascal Mognol, Jean-Yves Hascoet | 2014 | Robotics and Computer-Integrated Manufacturing30(2014)389–398. | State of the art methodologies | design methodology | detail/functional/manufacturability | ||||
| 2017 | 4 | Designing for Additive Manufacturing | B. Vayre, F. Vignat, F. Villeneuve | 2012 | Procedia CIRP 3 ( 2012 ) 632 – 637 | State of the art methodologies | design methodology | detail/concept/DFM/functional/innovation | ||||
| 2017 | 5 | Design for Additive Manufacturing—Element transitions and aggregated structures | Guido A.O. Adam, Detmar Zimmer | 2014 | CIRP Journal of Manufacturing Science and Technology 7 (2014) 20–28 | State of the art methodologies | design methodology | Design rules | ||||
| 2017 | 6 | Design and fabrication of reconstructive mandibular models using fused deposition modeling | Esfandyar Kouhi, Syed Masood and Yos Morsi | 2008 | Assembly Automation, Vol. 28, Issue 3 (2008), pp. 246–254. | State of the art methodologies | design methodology | customized/medicine/innovation | ||||
| 2017 | 7 | Development of a design feature database to support design for additive manufacturing | Shajahan Bin Maidin, Ian Campbell, Eujin Pei | 2012 | Assembly Automation, Vol. 32 Issue: 3,pp. 235-244 (2012) | State of the art methodologies | design methodology | Data: database/assembly/function/innovation | ||||
| 2017 | 8 | A rapid design and manufacturing system for product development applications | Wang Guangchun, Li Huiping, Guan Yanjin, Zhao Guoqun | 2004 | Rapid Prototyping Journal, Vol. 10 Issue: 3,pp. 200-206 | State of the art methodologies | design methodology | DFRM | ||||
| 2017 | 9 | Additive manufacturing-enabled design theory and methodology: a critical review | Sheng Yang & Yaoyao Fiona Zhao | 2015 | Int J Adv Manuf Technol (2015) 80:327–342 | State of the art methodologies | design methodology | state of the art | ||||
| 2017 | 10 | Integrated product-process design to suggest appropriate manufacturing technology: a review | Uzair Khaleeq uz Zaman, Ali Siadat, Mickael Rivette, Aamer Ahmed Baqai, Lihong Qiao, | 2017 | International Journal of Advanced Manufacturing Technology 91(1-4), pp. 1409-1430 | State of the art methodologies | design methodology | state of the art | ||||
| 2017 | 11 | Design for additive manufacturing based on the axiomatic design method | Konstantinos Salonitis | 2016 | Int J Adv Manuf Technol (2016) 87:989–996 | State of the art methodologies | design methodology | axiomatic/functional/restriction | ||||
| 2017 | 12 | Enriching design with X through tailored additive manufacturing knowledge: a methodological proposal | Laverne Floriane, Segonds Frédéric, D’Antonio Gianluca, Le Coq Marc | 2017 | International Journal on Interactive Design and Manufacturing 11(2), pp. 279-288 | State of the art methodologies | design methodology | DWAM/concept/functional/assembly/innovation | ||||
| 2017 | 13 | Holistic approach for industrializing AM technology: from part selection to test and verification | Thomas Reiher, Christian Lindemann, Ulrich Jahnke, Gereon Deppe, Rainer Koch | 2017 | Prog Addit Manuf 31 March, 2017 | State of the art methodologies | design methodology | holistic/function/assembly/manufacturability/innovation | ||||
| 2017 | 14 | An identification method for enclosed voids restriction in manufacturability design for additive manufacturing structures | Shutian LIU, Quhao LI, Wenjiong CHEN, Liyong TONG, Gengdong CHENG | 2015 | Front. Mech. Eng. 2015, 10(2): 126–137 | State of the art methodologies | design methodology | manufacturability | ||||
| 2017 | 15 | Additive Manufacturing of Metal Cellular Structures: Design and Fabrication | LI YANG, OLA HARRYSSON, DENIS CORMIER, HARVEY WEST, HAIJUN GONG and BRENT STUCKER | 2015 | JOM, Vol. 67, No. 3, 2015 | State of the art methodologies | design methodology | Analytical/experimental/cellular | ||||
| 2017 | 16 | A Design for Additive Manufacturing Ontology | Mahmoud Dinar, David W. Rosen | 2017 | Journal of Computing and Information Science in Engineering 17(2),021013 | State of the art methodologies | design methodology | Data: database/assembly/function/innovation | ||||
| 2017 | 17 | Additive Manufacturing to Advance Functional Design: An Application in the Medical Field | Claudio Comotti, Daniele Regazzoni, Caterina Rizzi, Andrea Vitali | 2017 | Journal of Computing and Information Science in Engineering 17(3),031006 | State of the art methodologies | design methodology | Function/doctor/innovation/state of the art | ||||
| 2017 | 18 | Assembly Based Methods to Support Product Innovation in Design for Additive Manufacturing: An Exploratory Case Study | Floriane Laverne, Frederic Segonds, Nabil Anwer, Marc Le Coq | 2015 | Journal of Mechanical Design, Transactions of the ASME 137(12),121701 | State of the art methodologies | design methodology | DWAM/concept/functional/assembly/innovation | ||||
| 2017 | 19 | Design and Analysis of Lattice Structures for Additive Manufacturing | Christiane Beyer, Dustin Figueroa | 2016 | Journal of Manufacturing Science and Engineering, Transactions of the ASME 138(12),121014 | State of the art methodologies | design methodology | experimental/cellular | ||||
| 2017 | 20 | Design for Additive Manufacture of Fine Medical Instrumentation: DragonFlex Case Study | Filip Jelınek, Paul Breedveld, | 2015 | Journal of Mechanical Design, Transactions of the ASME 137(11),111710 | State of the art methodologies | design methodology | functional/medical/innovation | ||||
| 2017 | 21 | An Investigation of Key Design for Additive Manufacturing Constraints in Multimaterial Three Dimensional Printing | Nicholas Meisel, Christopher Williams | 2015 | Journal of Mechanical Design, Transactions of the ASME 137(11),111703 | State of the art methodologies | design methodology | Design rules | ||||
| 2017 | 22 | Design Framework for Multifunctional Additive Manufacturing: Placement and Routing of Three-Dimensional Printed Circuit Volumes | A. Panesar, D. Brackett, I. Ashcroft, R. Wildman, R. Hague | 2015 | Journal of Mechanical Design, Transactions of the ASME 137(11),111708 | State of the art methodologies | design methodology | electronic/concept/single-objective optimization | ||||
| 2017 | 23 | Geometric Tailoring: A Design for Manufacturing Method for Rapid Prototyping and Rapid Tooling | Shiva Sambu, Yong Chen, David W. Rosen | 2004 | Journal of Mechanical Design, Transactions of the ASME 126(4), pp. 571-580 | State of the art methodologies | design methodology | experimental/multi-objective optimization | ||||
| 2017 | 24 | A Functional Classification Framework for the Conceptual Design of Additive Manufacturing Technologies | Christopher B. Williams, Farrokh Mistree, David W. Rosen | 2011 | Journal of Mechanical Design, Transactions of the ASME 133(12),121002 | State of the art methodologies | design methodology | concept/functional/process selection | ||||
| 2017 | 25 | Efficient Design-Optimization of Variable-Density Hexagonal Cellular Structure by Additive Manufacturing: Theory and Validation | Pu Zhang, Jakub Toman, Yiqi Yu, Emre Biyikli, Mesut Kirca, Markus Chmielus, Albert C. To | 2015 | Journal of Manufacturing Science and Engineering, Transactions of the ASME 137(2),021004 | State of the art methodologies | design methodology | Analytical/numerical experiment/real experiment/multiscale optimization | ||||
| 2017 | 26 | Integration of Design for Manufacturing Methods With Topology Optimization in Additive Manufacturing | Rajit Ranjan, Rutuja Samant, Sam Anand | 2017 | Journal of Manufacturing Science and Engineering, Transactions of the ASME 139(6),061007 | State of the art methodologies | design methodology | Manufacturability/topological optimization/multi-criteria optimization | ||||
| 2017 | 27 | DESIGN FOR ADDITIVE MANUFACTURING: INTERNAL CHANNEL OPTIMIZATION | M. Pietropaoli, R. Ahlfeld, F. Montomoli, A. Ciani, M. D’Ercole | 2017 | Journal of Engineering for Gas Turbines and Power 139(10),102101 | State of the art methodologies | design methodology | Topological optimization/single-objective optimization | ||||
| 2017 | 28 | Redesign and cost estimation of rapid manufactured plastic parts, | Eleonora Atzeni, Luca Iuliano, Paolo Minetola, Alessandro Salmi, | 2010 | Rapid Prototyping Journal, Volume 16 · Number 5 · 2010 · 308–317 | State of the art methodologies | design methodology | Function/assembly/cost | ||||
| 2017 | 29 | Systematic proposal to calculate price of prototypes manufactured through rapid prototyping an FDM 3D printer in a university lab | Carlos Henrique Pereira Mello, Rafael Calandrin Martins, Bruno Rosa Parra, Edson de Oliveira Pamplona, Eduardo Gomes Salgado, Rodrigo Tavares Seguso | 2010 | Rapid Prototyping Journal 16/6 (2010) 411–416 | State of the art methodologies | design methodology | cost | ||||
| 2017 | 30 | Speed and accuracy evaluation of additive manufacturing machines | Tomaz Brajlih, Bogdan Valentan, Joze Balic, Igor Drstvensek | 2011 | Rapid Prototyping Journal 17/1 (2011) 64–75 | State of the art methodologies | design methodology | design rules | ||||
| 2017 | 31 | Rapid casting solutions: a review | Munish Chhabra, Rupinder Singh | 2011 | Rapid Prototyping Journal, Vol. 17 (2011) Issue: 5,pp. 328-350 | State of the art methodologies | design methodology | state of the art. | ||||
| 2017 | 32 | A repository for DFM problems using description logics | Sungshik Yim, David W. Rosen | 2008 | Journal of Manufacturing Technology Management, Vol. 19 Issue: 6, (2008), pp. 755-774. | State of the art methodologies | design methodology | Data: database/assembly/manufacturability/guidelines/innovation | ||||
| 2017 | 33 | Computer‐aided build style decision support for stereolithography | Joel E. McClurkin, David W. Rosen | 1998 | Rapid Prototyping Journal, Vol. 4 Issue: 1, (1998) pp.4-13 | State of the art methodologies | design methodology | Tool for decision/experimental/multi-objective optimization. | ||||
| 2017 | 34 | 3D roughness profile model in fused deposition modelling | Alberto Boschetto, Veronica Giordano, Francesco Veniali | 2013 | Rapid Prototyping Journal 19(4),17088799, pp. 240-252 | State of the art methodologies | design methodology | Analytical model of roughness / experimental | ||||
| 2017 | 35 | A survey of the design methods for additive manufacturing to improve functional performance | Yunlong Tang, Yaoyao Fiona Zhao | 2016 | Rapid Prototyping Journal, Vol. 22 Issue: 3,pp. 569-590 | State of the art methodologies | design methodology | state of the art | ||||
| 2017 | 36 | Towards a sustainable and economic selection of part candidates for additive manufacturing | Christian Lindemann, Thomas Reiher, Ulrich Jahnke, Rainer Koch | 2015 | Rapid Prototyping Journal, Vol. 21 Issue: 2,pp. 216-227, | State of the art methodologies | design methodology | Selected part/function/assembly/topological optimization/cost | ||||
| 2017 | 37 | Selection of additive manufacturing processes | Yuanbin Wang, Robert Blache, Xun Xu, ( | 2017 | Rapid Prototyping Journal 23(2), pp. 434-447 | State of the art methodologies | design methodology | state of the art | ||||
| 2017 | 38 | On design for additive manufacturing: evaluating geometrical limitations | Guido A. O. Adam, Detmar Zimmer | 2015 | Rapid Prototyping Journal, Vol. 21 Issue: 6,pp. 662-670, | State of the art methodologies | design methodology | Design rules | ||||
| 2017 | 39 | Rapid prototyping process selection using multi criteria decisión making considering environmental criteria and its decision support system | Vimal KEK, Vinodh S., Brajesh P., Muralidharan R., | 2016 | Rapid Prototyping Journal, Vol. 22 Issue: 2,pp. 225-250 | State of the art methodologies | design methodology | process selection | ||||
| 2017 | 40 | Selection of selective laser sintering materials for different applications | Sunil Kumar Tiwari, Sarang Pande, Sanat Agrawal, Santosh M. Bobade | 2015 | Rapid Prototyping Journal, Vol. 21 Issue: 6,pp. 630-648, | State of the art methodologies | design methodology | material selection | ||||
| 2017 | 41 | A new global approach to design for additive manufacturing | R. Ponche , J.Y. Hascoet , O. Kerbrat & P. Mognol | 2012 | Virtual and Physical Prototyping, 7:2, 93-105 | State of the art methodologies | design methodology | DFAM/manufacturability/functionality/assemblability/restrictions | ||||
| 2017 | 42 | A new methodological framework for design for additive manufacturing | Martin Kumke, Hagen Watschke & Thomas Vietor | 2016 | Virtual and Physical Prototyping, 11:1, 3-19 | State of the art methodologies | design methodology | state of the art | ||||
| 2017 | 43 | An additive manufacturing process model for product family design | Ningrong Lei, Xiling Yao, Seung Ki Moon & Guijun Bi | 2016 | Journal of Engineering Design, 27:11, 751-767 | State of the art methodologies | design methodology | product family design | ||||
| 2017 | 44 | Design for Additive Manufacturing of Cellular Structures | Chen Chu, Greg Graf & David W. Rosen | 2008 | Computer-Aided Design and Applications, 5:5, 686-696 | State of the art methodologies | design methodology | Design and optimization at multiscale/innovation (software) | ||||
| 2017 | 45 | Design knowledge representation to support personalised additive manufacturing | Hyunwoong Ko, Seung Ki Moon & Kevin N. Otto | 2015 | Virtual and Physical Prototyping, 10:4, 217-226, | State of the art methodologies | design methodology | customization/software/innovation | ||||
| 2017 | 46 | Material and design considerations for rapid manufacturing | R. Hague , S. Mansour & N. Saleh | 2004 | International Journal of Production Research, 42:22, 4691-4708 | State of the art methodologies | design methodology | mechanical properties/design rules/design rules | ||||
| 2017 | 47 | Research supporting principles for design for additive manufacturing | David W. Rosen | 2014 | Virtual and Physical Prototyping, 9:4, 225-232 | State of the art methodologies | design methodology | state of the art | ||||
| 2017 | 48 | The status, challenges, and future of additive manufacturing in engineering | Wei Gao, Yunbo Zhang, Devarajan Ramanujan, Karthik Ramani, Yong Chen, Christopher B. Williams, Charlie C.L. Wang, Yung C. Shin, Song Zhang, Pablo D. Zavattieri | 2015 | Computer-Aided Design 69 (2015) 65–89 | State of the art methodologies | design methodology | state of the art | ||||
| 2017 | 49 | Design and manufacture of high performance hollow engine valves by Additive Layer Manufacturing | D. Cooper J. Thornby, N. Blundell, R. Henrys, M.A. Williams, G. Gibbons | 2015 | Materials and Design 69 (2015) 44–55 | State of the art methodologies | design methodology | Case study/topological optimization/reverse engineering | ||||
| 2017 | 50 | Additive manufacturing: Toward holistic design | Bradley H. Jared ⁎, Miguel A. Aguilo, Lauren L. Beghini, Brad L. Boyce, BrettW. Clark, Adam Cook, Bryan J. Kaehr, Joshua Robbins | 2017 | Scripta Materialia 135, pp. 141-147 | State of the art methodologies | design methodology | state of the art | ||||
| 2017 | 51 | A new part consolidation method to embrace the design freedom of additive manufacturing | Sheng Yang, Yunlong Tang, Yaoyao Fiona Zhao | 2015 | Journal of Manufacturing Processes 20 (2015) 444–449 | State of the art methodologies | design methodology | Function/assembly/topological | ||||
| 2017 | 52 | Grouping parts for multiple parts production in Additive Manufacturing | Yicha Zhang*, Alain Bernard | 2014 | Procedia CIRP 17 ( 2014 ) 308 – 313 | State of the art methodologies | design methodology | manufacturability | ||||
| 2017 | 53 | Design for Additive Manufacturing – Supporting the Substitution of Components in Series Products | Christoph Klahn*, Bastian Leutenecker, Mirko Meboldt | 2014 | Procedia CIRP 21 ( 2014 ) 138 – 143 | State of the art methodologies | design methodology | Functionality/assembly/customization | ||||
| 2017 | 54 | Evaluating the Design for Additive Manufacturing: A Process Planning Perspective | Yicha Zhanga, Alain Bernard, Ravi Kumar Gupta, Ramy Harik | 2014 | Procedia CIRP 21 ( 2014 ) 144 – 150 | State of the art methodologies | design methodology | Function/manufacturability/process | ||||
| 2017 | 55 | Design Strategies for the Process of Additive Manufacturing | Christoph Klahn, Bastian Leutenecker, Mirko Meboldt | 2015 | Procedia CIRP 36 ( 2015 ) 230 – 235. | State of the art methodologies | design methodology | functionality | ||||
| 2017 | 56 | Towards Annotations and Product Definitions for Additive Manufacturing | Paul Witherell, Jennifer Herron, Gaurav Ameta | 2016 | Procedia CIRP 43 ( 2016 ) 339 – 344 | State of the art methodologies | design methodology | State of the art (dimensional and geometric tolerances) | ||||
| 2017 | 57 | Design Guidelines for Additive Manufactured Snap-Fit Joints | Christoph Klahn, Daniel Singer, Mirko Meboldt | 2016 | Procedia CIRP 50 ( 2016 ) 264 – 269 | State of the art methodologies | design methodology | Functional/assembly/joints | ||||
| 2017 | 58 | (Re)Design for Additive Manufacturing | Sebastian Hällgren, Lars Pejryd, Jens Ekengren | 2016 | Procedia CIRP 50 ( 2016 ) 246 – 251. | State of the art methodologies | design methodology | Manufacturability/multiscale optimization | ||||
| 2017 | 59 | Considering Part Orientation in Design for Additive Manufacturing | Bastian Leutenecker-Twelsiek, Christoph Klahn, Mirko Meboldt | 2016 | Procedia CIRP 50 ( 2016 ) 408 – 413 | State of the art methodologies | design methodology | manufacturabilidad/metodo de decision translated into English is manufacturability/decision method. | ||||
| 2017 | 60 | A design framework to replace conventional manufacturing processes with additive manufacturing for structural components: A formula student case study | Harry Bikas, John Stavridis, Panagiotis Stravropoulos, George Chryssolouris | 2016 | , Procedia CIRP 57 (2016) 710-715. | State of the art methodologies | design methodology | ensemble/functionality | ||||
| 2017 | 61 | Design for Rapid Manufacturing functional SLS parts | Walter Kruf M. Sc., Bart van de Vorst B. Sc., Hessel Maalderink B. Sc., Nico Kamperman M. Sc, | 2006 | Intelligent Production Machines and Systems (2006) | State of the art methodologies | design methodology | functional | ||||
| 2017 | 62 | Additive manufacturing and sustainability: an exploratory study of the advantages and challenges | Simon Ford, M_elanie Despeisse | 2016 | Journal of Cleaner Production 137 (2016) 1573-1587 | State of the art methodologies | design methodology | state of the art. | ||||
| 2017 | 63 | Additive Manufacturing for product improvement at Red Bull Technology | David E. Cooper, Mark Stanford, Kevin A. Kibble, Gregory J. Gibbons | 2012 | Materials and Design 41 (2012) 226–230 | State of the art methodologies | design methodology | case study | ||||
| 2017 | 64 | Additive Manufacturing of Custom Orthoses and Prostheses – A Review | Yu-an Jin, Jeff Plott, Roland Chen, Jeffrey Wensman, Albert Shih | 2015 | Procedia CIRP 36 ( 2015 ) 199 – 204 | State of the art methodologies | design methodology | state of the art. | ||||
| 2017 | 65 | A new Steiner patch based file format for Additive Manufacturing Processes | Ratnadeep Paul, Sam Anand | 2015 | Computer-Aided Design 63 (2015) 86–100 | State of the art methodologies | design methodology | New CNC format | ||||
| 2017 | 66 | A voxel-based method of constructing and skinning conformal and functionally graded lattice structures suitable for additive manufacturing | A.O. Aremu, J.P.J. Brennan-Craddock, A. Panesar, I.A. Ashcroft∗, R.J.M. Hague, R.D. Wildman, | 2017 | Additive Manufacturing 13 (2017) 1–13 | State of the art methodologies | design methodology | optimization grid | ||||
| 2017 | 67 | Build Orientation Determination for Multi-material Deposition Additive Manufacturing with Continuous Fibers | Yicha Zhang, Wout De Backer, Ramy Harik, Alain Bernard | 2016 | Procedia CIRP 50 ( 2016 ) 414 – 419 | State of the art methodologies | design methodology | manufacturability | ||||
| 2017 | 68 | Characterization of effect of support structures in laser additive manufacturing of stainless Steel | Jukka-Pekka Järvinen, Ville Matilainen, Xiaoyun Li, Heidi Piili, Antti Salminen, Ismo Mäkelä, Olli Nyrhilä | 2014 | Physics Procedia 56 ( 2014 ) 72 – 81 | State of the art methodologies | design methodology | Support/manufacturability/design rule | ||||
| 2017 | 69 | A framework to reduce product environmental impact through design optimization for additive manufacturing | Yunlong Tang, Kieran Mak, Yaoyao Fiona Zhao | 2016 | Journal of Cleaner Production 137 (2016) 1560e1572. | State of the art methodologies | design methodology | environmental impact | ||||
| 2017 | 70 | Generalized requirements and decompositions for the design of test parts for micro additive manufacturing research | Mary Kathryn Thompson, and Line Harder Clemmensen | 2015 | Procedia CIRP 34 ( 2015 ) 229 – 235 | State of the art methodologies | design methodology | functional | ||||
| 2017 | 71 | Geometric consideration of support structures in part overhang fabrications by electron beam additive manufacturing | Bo Cheng, Kevin Chou | 2015 | Computer-Aided Design 69 (2015) 102–111 | State of the art methodologies | design methodology | Manufacturability/design rule | ||||
| 2017 | 72 | Permeability and strength of a porous metal structure fabricated by additive manufacturing | Tatsuaki Furumoto, Ayato Koizumi, Mohd Rizal Alkahari, Rui Anayama, Akira Hosokawa, Ryutaro Tanaka, Takashi Ueda | 2015 | Journal of Materials Processing Technology 219 (2015) 10–16 | State of the art methodologies | design methodology | Function/design rule | ||||
| 2017 | 73 | Redesign Optimization for Manufacturing Using Additive Layer Techniques | Konstantinos Salonitis, Saeed Al Zarban | 2015 | Procedia CIRP 36 ( 2015 ) 193 – 198 | State of the art methodologies | design methodology | Functionality/optimization | ||||
| 2017 | 74 | Towards early estimation of part accuracy in additive manufacturing | Giovanni Moroni, Wahyudin P. Syam, Stefano Petr`o | 2014 | Procedia CIRP 21 ( 2014 ) 300 – 305 | State of the art methodologies | design methodology | Manufacturability/design rules | ||||
| 2017 | 75 | Bidirectional Evolutionary Structural Optimization (BESO) based design method for lattice structure to be fabricated by additive manufacturing | Yunlong Tang, Aidan Kurtz, Yaoyao Fiona Zhao | 2015 | Computer-Aided Design 69 (2015) 91–101 | State of the art methodologies | design methodology | cellular function/optimization and lattice | ||||
| 2017 | 76 | Identification of optimal printing conditions for laser printing of alginate tubular constructs | Ruitong Xiong, Zhengyi Zhang, Yong Huang | 2015 | Journal of Manufacturing Processes 20 (2015) 450–455 | State of the art methodologies | design methodology | Manufacturability/design rules | ||||
| 2017 | 77 | Design for additive manufacturing: Automated build orientation selection and optimization | Marijn P. Zwier and Wessel W. Wits | 2016 | Procedia CIRP 55 ( 2016 ) 128 – 133 | State of the art methodologies | design methodology | Manufacturability/optimization of orientation | ||||
| 2017 | 78 | Two-dimensional placement optimization for multi-parts production in additive manufacturing | Yicha Zhang, RaviKumar Gupta , Alain Bernard | 2016 | Robotics and Computer-Integrated Manufacturing 38 (2016) 102–117 | State of the art methodologies | design methodology | Manufacturability/optimization of orientation | ||||
| 2017 | 79 | Optimal topology for additive manufacture: A method for enabling additive manufacture of support-free optimal structures | Martin Leary, Luigi Merli, Federico Torti, Maciej Mazur, Milan Brandt | 2014 | Materials and Design 63 (2014) 678–690 | State of the art methodologies | design methodology | manufacturability/function/optimization | ||||
| 2017 | 80 | Topology optimization of 3D self-supporting structures for additive manufacturing | Matthijs Langelaar | 2016 | Additive Manufacturing 12 (2016) 60–70 | State of the art methodologies | design methodology | manufacturability/function/optimization | ||||
| 2017 | 81 | An Application Specific Additive Design Methodology for the Determination of Heatsink Geometry Topologies | Robin Bornoff, John Parry | 2015 | Therminic 2015, 21st INTERNATIONAL WORKSHOP on Thermal Investigations of ICs and Systems, September / October 2015, Paris / FR | State of the art methodologies | design methodology | function/optimization | ||||
| 2017 | 82 | DESIGN OF LATTICE STRUCTURE FOR ADDITIVE MANUFACTURING | Wenjin Tao, Ming C. Leu | 2016 | Proceedings of ISFA2016, 2016 International Symposium on Flexible Automation Cleveland, Ohio, U.S.A., 1 - 3 August, 2016 | State of the art methodologies | design methodology | State of the art (cell and lattice design) | ||||
| 2017 | 82 B | Design and additive manufacturing of cellular lattice structures | Hao, L., Raymont, D., Yan, C., Hussein, A., Young, P. | 2011-2012 | Innovative Developments in Virtual and Physical Prototyping - Proceedings of the 5th International Conference on Advanced Research and Rapid Prototyping pp. 249-254 | State of the art methodologies | design methodology | State of the art (cell and lattice design) | ||||
| 2017 | 83 | Influences of Additive Manufacturing on Design Processes for Customised Products | Dieter Krause, Johanna Spallek, Olga Sankowski | 2016 | Conference Paper · May 2016, INTERNATIONAL DESIGN CONFERENCE - DESIGN 2016 Dubrovnik - Croatia, May 16 - 19, 2016 | State of the art methodologies | design methodology | State of the art (personalization) | ||||
| 2017 | 84 | Design Optimization Method for Additive Manufacturing of the Primary Mirror of a Large-Aperture Space Telescope | Rui Hu; Wenjiong Chen, Quhao Li; Shutian Liu; Ping Zhou, Zhigang Dong; and Renke Kang | 2017 | J. Aerosp. Eng., 2017, 30(3): 04016093 | State of the art methodologies | design methodology | Case study (functional/optimization) | ||||
| 2017 | 85 | Design and Additive Manufacturing of Periodic Ceramic Architectures | G. Bianchi, S. Gianella, A. Ortona | 2017 | J. Ceram. Sci. Tech., 08 [01] 59-66 (2017). | State of the art methodologies | design methodology | function/innovation | ||||
| 2017 | 86 | Implementation of the additive technology to the design and manufacturing of vibroisolators with required filtering | V.G. Smelov, A.V. Sotov, A.V. Agapovichev, M.M. Laktionova, T.M. Tomilina | 2017 | Procedia Engineering 176 ( 2017 ) 540 – 545 | State of the art methodologies | design methodology | Case study (function) | ||||
| 2017 | 87 | A Low-Cost Environmental Monitoring System: How to Prevent Systematic Errors in the Design Phase through the Combined Use of Additive Manufacturing and Thermographic Techniques | Francesco Salamone *, Ludovico Danza, Italo Meroni and Maria Cristina Pollastro | 2017 | Sensors 2017, 17, 828; doi:10.3390/s17040828 | State of the art methodologies | design methodology | Case study (function) | ||||
| 2017 | 88 | An improved lattice structure design optimization framework considering additive manufacturing constraints | Recep M. Gorguluarslan, Umesh N. Gandhi, Yuyang Song, Seung-Kyum Choi | 2017 | Rapid Prototyping Journal, Vol. 23 Issue: 2, pp.305-319, | State of the art methodologies | design methodology | Function/manufacturability/optimization | ||||
| 2017 | 89 | Lattice Structure Design and Optimization With Additive Manufacturing Constraints | Yunlong Tang, Guoying Dong, Qinxue Zhou, and Yaoyao Fiona Zhao | 2017 | IEEE TRANSACTIONS ON AUTOMATION SCIENCE AND ENGINEERING, 2017 | State of the art methodologies | design methodology | Function/manufacturability/optimization | ||||
| 2017 | 90 | Design framework for multifunctional additive manufacturing: Coupled optimization strategy for structures with embedded functional systems | Ajit Panesar∗, Ian Ashcroft, David Brackett, Ricky Wildman, Richard Hague | 2017 | Additive Manufacturing 16 (2017) 98–106 | State of the art methodologies | design methodology | function/optimization | ||||
| 2017 | 91 | Information exchange standards for design, tolerancing and Additive Manufacturing: a research review | Jinhua Xiao, Nabil Anwer, Alexandre Durupt, Julien Le Duigou, Benoît Eynard | 2017 | , Int J Interact Des Manuf Received: 12 March 2017 / Accepted: 3 May 2017 © Springer-Verlag France 2017 | State of the art methodologies | design methodology | State of the art (tolerance standards) | ||||
| 2017 | 92 | Bond interface design for single lap joints using polymeric additive manufacturing | R. Garcia, P. Prabhakar | 2017 | Composite Structures 176 (2017) 547–555 | State of the art methodologies | design methodology | Design of joints in assemblies | ||||
| 2017 | 93 | Design for additive manufacturing method for a mechanical system downsizing | Myriam Orquéra*, Sébastien Campocasso, Dominique Millet | 2017 | Procedia CIRP 60 ( 2017 ) 223 – 228 | State of the art methodologies | design methodology | function/assembly | ||||
| 2017 | 94 | Analysis of Design Guidelines for Automated Order Acceptance in Additive Manufacturing | Jan-Peer Rudolph, Claus Emmelmann | 2017 | Procedia CIRP 60 ( 2017 ) 187 – 192 | State of the art methodologies | design methodology | manufacturability | ||||
| 2017 | 95 | Integrated Design For Additive Manufacturing based on Skin-Skeleton Approach | Elnaz Asadollahi-Yazdi, Julien Gardan, Pascal Lafon | 2017 | Procedia CIRP 60 ( 2017 ) 217 – 222 | State of the art methodologies | design methodology | Functionality/manufacturability | ||||
| 2017 | 96 | DfAM in the design process: a proposal of classification to foster early design stages. | Laverne, F., et al. | 2014 | Confere. Sibenik, Croatia | State of the art methodologies | design methodology | state of the art. | ||||
| 2017 | 97 | Self-supporting structure design in additive manufacturing through explicit topology optimization | Guo, X., Zhou, J., Zhang, W., (...), Liu, C., Liu, Y. | 2017 | Computer Methods in Applied Mechanics and Engineering 323, pp. 27-63 | State of the art methodologies | design methodology | manufacturability/function/optimization | ||||
| 2017 | 98 | Additive Manufacturing-Oriented Design of Graded Lattice Structures Through Explicit Topology Optimization | Liu, C., Du, Z., Zhang, W., Zhu, Y., Guo, X. | 2017 | Journal of Applied Mechanics, Transactions ASME 84(8),081008 | State of the art methodologies | design methodology | Function/multiscale optimization | ||||
| 2019 | 99 | Multidisciplinary design optimization to identify additive manufacturing resources in customized product development | Yao, X., Moon, S.K., Bi, G. | 2017 | Journal of Computational Design and Engineering 4(2), pp. 131-142 | State of the art methodologies | design methodology | functional/customization/innovation | ||||
| 2019 | 100 (O29) | Support structure design in additive manufacturing based on topology optimization | Kuo, Y.-H., Cheng, C.-C., Lin, Y.-S., San, C.-H. | 2017 | Structural and Multidisciplinary Optimization pp. 1-13 | State of the art methodologies | design methodology | function/optimization | ||||
| 2019 | 101 | An improved methodology for design of custom-made hip prostheses to be fabricated using additive manufacturing technologies | Sadegh Rahmati, Farid Abbaszadeh, Farzam Farahmand | 2012 | Rapid Prototyping Journal, Vol. 18 Issue: 5,pp. 389-400 | State of the art methodologies | design methodology | Function/custom/medicine/innovation | ||||
| 2019 | 102 | Selecting parts for additive manufacturing in service logistics | Nils Knofius, Matthieu C. van der Heijden, W.H.M. Zijm | 2016 | Journal of Manufacturing Technology Management, Vol. 27 Issue: 7,pp. 915-931 | State of the art methodologies | design methodology | economy | ||||
| 2019 | 103 | A new process for design and manufacture of tailor-made functionallygraded composites through friction stir additive manufacturing | Sharma, A., Bandari, V., Ito, K., (...), Ramji, R.M., Himasekhar, H.S. | 2017 | Journal of Manufacturing Processes 26, pp. 122-130 | State of the art methodologies | design methodology | New design and manufacturing method AM/innovation/design rule | ||||
| 2019 | 104 | Interactive design of dental implant placements through CAD-CAM technologies: from 3D imaging to additive manufacturing | Barone, S., Casinelli, M., Frascaria, M., Paoli, A., Razionale, A.V. | 2016 | International Journal on Interactive Design and Manufacturing 10(2), pp. 105-117 | State of the art methodologies | design methodology | customization/medicine/innovation | ||||
| 2019 | 105 | An additive manufacturing filter for topology optimization of print-ready designs | Langelaar, M. | 2017 | Structural and Multidisciplinary Optimization 55(3), pp. 871-883 | State of the art methodologies | design methodology | Function/topological optimization/restriction | ||||
| 2019 | 106 | Additive manufacturing integration with topology optimization methodology for innovative product design | Primo, T., Calabrese, M., Del Prete, A., Anglani, A. | 2017 | International Journal of Advanced Manufacturing Technology pp. 1-13 | State of the art methodologies | design methodology | function/multiscale optimization/innovation | ||||
| 2019 | 107 ( 310) | Topology optimization considering overhang constraints: Eliminating sacrificial support material in additive manufacturing through design | Gaynor, A.T., Guest, J.K. | 2016 | Structural and Multidisciplinary Optimization 54(5), pp. 1157-1172 | State of the art methodologies | design methodology | Function/topological optimization/restriction | ||||
| 2019 | 108 | A design, mechanical rating, and load adaptation method for cellular components for additive manufacturing | Ziegler, T., Jaeger, R., Koplin, C. | 2017 | International Journal of Advanced Manufacturing Technology 90(9-12), pp. 2875-2884 | State of the art methodologies | design methodology | Function/Cellular optimization | ||||
| 2019 | 109 | A new approach to the design and optimisation of support structures in additive manufacturing | Strano, G., Hao, L., Everson, R.M., Evans, K.E. | 2013 | International Journal of Advanced Manufacturing Technology 66(9-12), pp. 1247-1254 | State of the art methodologies | design methodology | Function/optimization/manufacturability | ||||
| 2019 | 110 | Topology optimization for hybrid additive-subtractive manufacturing | Liu, J., To, A.C. | 2017 | Structural and Multidisciplinary Optimization 55(4), pp. 1281-1299 | State of the art methodologies | design methodology | Function/optimization/hybrid manufacturing | ||||
| 2019 | 111 | Customised design and manufacture of protective face masks combining a practitioner-friendly modelling approach and low-cost devices for digitising and additive manufacturing | Cazon, A., Aizpurua, J., Paterson, A., Bibb, R., Campbell, R.I. | 2014 | Virtual and Physical Prototyping 9(4), pp. 251-261 | State of the art methodologies | design methodology | function/custom/reverse engineering | ||||
| 2019 | 112 | Cross-sectional Structural Analysis for 3D Printing Optimization | Umetani, N., Schmidt, R. | 2013 | SIGGRAPH Asia 2013 Technical Briefs, SA 2013 5 | State of the art methodologies | design methodology | function/optimization | ||||
| 2019 | 113 | Materializing design: the implications of rapid prototyping in digital design | Larry Sass | 2006 | Design Studies Vol 27 No. 3 May 2006 | State of the art methodologies | design methodology | Architecture | ||||
| 2017 | 114 | Design for Additive Manufacturing: Trends, opportunities, considerations, and constraints | Mary Kathryn Thompson a,*, Giovanni Moroni (2)b, Tom Vaneker (2)c, Georges Fadel d, R. Ian Campbell e, Ian Gibson f, Alain Bernard (1)g, Joachim Schulz (3)h, Patricia Graf h, Bhrigu Ahuja i, Filomeno Martina | 2016 | CIRP Annals - Manufacturing Technology 65(2), pp. 737-760 | State of the art methodologies | design methodology | state of the art. | ||||
| 2017 | 115 | 3D printing with polymers: Challenges among expanding options and opportunities | Jeffrey W. Stansburya,b,∗, Mike J. Idacavage | 2016 | d e n t a l m a t e r i a l s 3 2 ( 2 0 1 6 ) 54–64 | State of the art methodologies | methodology | state of the art. | ||||
| 2019 | 116 | The additive manufacturing innovation: a range of implications | Harm-Jan Steenhuis, Leon Pretorius | 2017 | Journal of Manufacturing Technology Management, Vol. 28 Issue: 1, pp.122-143 | State of the art methodologies | methodology | State of the art/innovation | ||||
| 2019 | 117 | A scientometric review of hotspots and emerging trends in additive manufacturing | Yuran Jin, Shoufeng Ji, Xin Li, Jiangnan Yu | 2017 | Journal of Manufacturing Technology Management, Vol. 28 Issue: 1, pp.18-38 | State of the art methodologies | methodology | state of the art. | ||||
| 2019 | 118 | Integrated product-process design: Material and manufacturing process selection for additive manufacturing using multi-criteria decision making | Uzair Khaleeq uz Zaman a , ∗ , Mickael Rivette a , Ali Siadat a , Seyed Meysam Mousavi b | 2018 | Robotics and Computer–Integrated Manufacturing 51 (2018) 169–180 | State of the art methodologies | methodology | material selection and processes | ||||
| 2019 | 119 (161) | Design methodology for porous composites with tunable thermal expansion produced by multi-material topology optimization and additive manufacturing | Akihiro Takezawa a, *, Makoto Kobashi b | 2017 | Composites Part B 131 (2017) 21e29 | State of the art methodologies | methodology | thermal function | ||||
| 2019 | 120 | Metal additive manufacturing of a high-pressure micro-pump | Wessel W. Witsa,*, Sander J. Weitkampa, Johannes van Esb | 2013 | Procedia CIRP 7 ( 2013 ) 252 – 257 | State of the art methodologies | methodology | Function (fluid mechanics), (case study) | ||||
| 2019 | 121 | Process Planning for the Fuse Deposition Modeling of Ankle-Foot-Othoses | Yuan Jina,b* , Yong Heb, Albert Shiha,c | 2016 | Procedia CIRP 42 ( 2016 ) 760 – 765 | State of the art methodologies | methodology | function/customization/medicine | ||||
| 2019 | 122 (169) | Systematic Biomimetic Part Design for Additive Manufacturing | Tobias Kamps*a, Melanie Gralowa, Georg Schlicka, Gunther Reinhart a, b | 2017 | Procedia CIRP 65 ( 2017 ) 259 – 266 | State of the art methodologies | methodology | Function (resistance and weight) / optimization | ||||
| 2019 | 123 (168) | Cloud-based Design and Additive Manufacturing of Custom Orthoses | Shih, A., Park, D.W., Yang, Y.-Y., Chisena, R., Wu, D | 2017 | Procedia CIRP , 63 pp. 156 - 160 | State of the art methodologies | methodology | function/customization/medicine/innovation | ||||
| 2019 | 124 (341) | An additive manufacturing oriented design approach to mechanical assemblies | Germain Sossou, Frédéric Demoly ⇑, Ghislain Montavon, Samuel Gomes | 2018 | Journal of Computational Design and Engineering 5 (2018) 3–18 | State of the art methodologies | methodology | Function (mechanics)/assembly | ||||
| 2019 | 125 | Fundamentals of mechanical design and analysis for AM fabricated parts | Alexandre V. Manzhirov | 2017 | Procedia Manufacturing 7 (2017) 59-65 | State of the art methodologies | methodology | function (mechanics) | ||||
| 2019 | 126 (176) | Physical Rigging for Physical Models and Posable Joint Designs Based on Additive Manufacturing Technology | Yingtian Li, Yonghua Chen* | 2017 | Procedia Manufacturing 11 ( 2017 ) 2235 – 2242 | State of the art methodologies | methodology | Function (mechanics)/assembly | ||||
| 2019 | 127 (342) | Design for manufacturing to design for Additive Manufacturing: Analysis of implications for design optimality and product sustainability | Gebisa, A.W., Lemu, H.G. | 2017 | Procedia Manufacturing, 13, pp. 724-731. | State of the art methodologies | methodology | Function/optimization/sustainability | ||||
| 2019 | 128 (153) | Methods and tools for identifying and leveraging additive manufacturing design potentials | Martin Kumke1 · Hagen Watschke2 · Peter Hartogh2 · Ann-Kathrin Bavendiek2 · Thomas Vietor2 | 2017 | Int J Interact Des Manuf, Received: 10 March 2017 / Accepted: 18 April 2017 | State of the art methodologies | methodology | function/optimization/innovation | ||||
| 2019 | 129 (151) | A methodological proposal to link Design with Additive Manufacturing to environmental considerations in the Early Design Stages | Foteini MarkouFrédéric SegondsMaud RioNicolas Perry | 2017 | International Journal on Interactive Design and Manufacturing (IJIDeM) November 2017, Volume 11, Issue 4, pp 799–812 | State of the art methodologies | methodology | Environment | ||||
| 2019 | 130 | Interactive design for additive manufacturing: a creative case of synchronous belt drive | Hu Fuwen · Cheng Jiajian · He Yunhua | 2017 | International Journal on Interactive Design and Manufacturing, pp. 1-13. | State of the art methodologies | methodology | function (case study) / innovation | ||||
| 2019 | 131 ( 180) | Design for additive manufacturing of customized cast with porous shell structures | Yeong-Eun Lim, Na-Hyun Kim, Hye-Jin Choi and Keun Park | 2017 | Journal of Mechanical Science and Technology 31 (11) (2017) 5477~5483 | State of the art methodologies | methodology | function/customization/medicine/innovation | ||||
| 2019 | 132 | DESIGN RULES FOR ADDITIVE MANUFACTURING: A CATEGORIZATION | Mahesh Mani, Paul Witherell, Jacob Jee | 2017 | Proceedings of the ASME Design Engineering Technical Conference, 1, art. no. 68446. | State of the art methodologies | methodology | design rules | ||||
| 2019 | 133 ( 178) | Which material design is possible under additive manufacturing: A fuzzy approach | Zapata, F., Kosheleva, O., Kreinovich, V. | 2017 | IFSA-SCIS 2017 - Joint 17th World Congress of International Fuzzy Systems Association and 9th International Conference on Soft Computing and Intelligent Systems, art. no. 8023228. | State of the art methodologies | methodology | material selection | ||||
| 2019 | 134 | Augmenting Computer-Aided Design Software with Multi-Functional Capabilities to Automate Multi-Process Additive Manufacturing | Callum Bailey, Efrain Aguilera, David Espalin, Jose Motta, Alfonso Fernandez, Mireya A. Perez, Christopher DiBiasio, Dariusz Pryputniewicz, Eric MacDonald, Ryan B. Wicker | 2017 | IEEE Access. | State of the art methodologies | methodology | systems of design and manufacturing | ||||
| 2019 | 135 | An Overview on Additive Manufacturing of Polymers | Iwona Jasiuk, Diab W. Abueidda, Christopher Kozuch, Siyuan Pang, Frances Y. Su & Joanna McKittrick | 2018 | the journal of the Minerals, Metals & Materials Society · January 2018 | State of the art methodologies | state of the art. | state of the art. | ||||
| 2019 | 136 ( 186) | New to Power Equipment Design Approaches with Additive Manufacturing prospects | O V Belova and M D Vulf | 2017 | Journal of Physics: Conference Series, Volume 891, conference 1 | State of the art methodologies | methodology | Function (thermofluids), (case studies), state of the art. | ||||
| 2019 | 137 | Part separation methods for assembly based design in additive manufacturing | Oh, Y., Behdad, S., Zhou, C. | 2017 | Proceedings of the ASME Design Engineering Technical Conference | State of the art methodologies | methodology | Function/assembly/optimization | ||||
| 2019 | 138 ( 355) | FDM for Composite Tooling DESIGN GUIDE | stratasys | - | libro | State of the art methodologies | methodology | Function (tools, case studies) / design rules and guidelines. | ||||
| 2019 | 139 | Democratizing science with the aid of parametric design and additive manufacturing: Design and fabrication of a versatile and low-cost optical instrument for scattering measurement | Jose M. Nadal-Serrano1☯*, Adolfo Nadal-Serrano2☯, Marisa Lopez-Vallejo1³ | 2017 | PLoS ONE 12(11): e0187219. | State of the art methodologies | methodology | function (electronics and optics)/innovation | ||||
| 2019 | 140 ( 170) | Design and Performance Assessment of Innovative Eco-Efficient Support Structures for Additive Manufacturing by Photopolymerization | Andre´s D´ıaz Lantada , Adria´n de Blas Romero, A´ lvaro Sa´nchez Isasi, and Diego Garrido Bellido | 2017 | Journal of Industrial Ecology, Volume 21, Number S1 | State of the art methodologies | methodology | Function (mechanics and weight)/Optimization/innovation | ||||
| 2019 | 141 ( 165) | Design for Additive Manufacturing, to produce assembled products, by SLS | Nicolae Bâlc1,*, and Cristian Vilău1 | 2017 | MATEC Web of Conferences 121, 04002 (2017) | State of the art methodologies | methodology | ensemble | ||||
| 2019 | 142 | Redesigning a Reaction Control Thruster for Metal-Based Additive Manufacturing: A Case Study in Design for Additive Manufacturing | Nicholas A. Meisel, Matthew R. Woods, Timothy W. Simpson, Corey J. Dickman | 2017 | Journal of Mechanical Design, OCTOBER 2017, Vol. 139 | State of the art methodologies | methodology | Function (thermofluids), (case studies) | ||||
| 2019 | 143 | Power–Velocity Process Design Charts for Powder Bed Additive Manufacturing | Daniel R. Clymer, Jonathan Cagan, Jack Beuth | 2017 | Journal of Mechanical Design, OCTOBER 2017, Vol. 139 | State of the art methodologies | methodology | Design rule (performance cards) | ||||
| 2019 | 144 | Parametric Design of Scalable Mechanisms for Additive Manufacturing | Li, X., Zhao, J., He, R., Tian, Y., Wei, X. | 2018 | Journal of Mechanical Design, Transactions of the ASME, 140(2), art. no. 022302. | State of the art methodologies | methodology | Function (mechanisms) | ||||
| 2019 | 145 | The design formulae for skew line gear wheel structures oriented to the additive manufacturing technology based on strength analysis | Lyu, Y., Chen, Y., Lin, Y. | 2017 | Mechanical Sciences, 8(2), pp. 369-383. | State of the art methodologies | methodology | Function (mechanics and mechanisms)/manufacturing (orientation) | ||||
| 2019 | 146 ( 185) | A method for modularity in design rules for additive manufacturing | Haeseong Jee, Paul Witherell | 2017 | Rapid Prototyping Journal, Vol. 23 Issue: 6, pp.1107-1118, | State of the art methodologies | methodology | ensemble | ||||
| 2019 | 147 ( 184) | A hybrid machine learning approach for additive manufacturing design feature recommendation | Xiling Yao, Seung Ki Moon, Guijun Bi, | 2017 | Rapid Prototyping Journal, Vol. 23 Issue: 6, pp.983-997 | State of the art methodologies | methodology | Design rule (neural network program) | ||||
| 2019 | 148 | Assembly Design Framework for Additive Manufacturing (AM) based on Axiomatic Design (AD) | Yosep Oh, Sara Behdad | 2017 | Proceedings of the 2017 Industrial and Systems Engineering Conference | State of the art methodologies | methodology | ensemble | ||||
| 2019 | 149 | Rate limits of additive manufacturing by fused filament fabrication and guidelines for high-throughput system design | Jamison Goa, Scott N. Schiffres a,b, Adam G. Stevensa, A. John Harta,∗ | 2017 | Additive Manufacturing 16 (2017) 1–11 | State of the art methodologies | methodology | Design rules, comparison of industrial and desktop machines | ||||
| 2019 | 150 | Security features embedded in computer aided design (CAD) solid models for additive manufacturing | Fei Chen and Gary Mac and Nikhil Gupta | 2017 | Materials & Design 128 (2017) 182–194 | State of the art methodologies | methodology | Copyright, design to protect copyright | ||||
| 2019 | 151 ( 129) | A methodological proposal to link Design with Additive Manufacturing to environmental considerations in the Early Design Stages | Foteini Markou and Fr{\'{e}}d{\'{e}}ric Segonds and Maud Rio and Nicolas Perry | 2017 | Int J Interact Des Manuf | State of the art methodologies | methodology | environment | ||||
| 2019 | 152 ( 199) | Additive Manufacturing: Rethinking Battery Design | C. L. Cobb and C. C. Ho | 2016 | Interface magazine | State of the art methodologies | methodology | Function (electric), design rules | ||||
| 2019 | 153 ( 128) | Methods and tools for identifying and leveraging additive manufacturing design potentials | Martin Kumke and Hagen Watschke and Peter Hartogh and Ann-Kathrin Bavendiek and Thomas Vietor | 2018 | Int J Interact Des Manuf (2018) 12:481–493 | State of the art methodologies | methodology | utilization | ||||
| 2019 | 154 | Design Optimization of Plastic Injection Tooling for Additive Manufacturing | Tong Wu and Suchana A. Jahan and Yi Zhang and Jing Zhang and Hazim Elmounayri and Andres Tovar | 2017 | Procedia Manufacturing 10 ( 2017 ) 923 – 934 | State of the art methodologies | methodology | Optimization, thermofluids, topological, lattice | ||||
| 2019 | 155 | A design tool for resource-efficient fabrication of 3d-graded structura building components using additive manufacturing | F. Craveiroa, H.M. Bartoloa,b, A. Galec, J.P. Duartea,d, P.J. Bartoloa,c | 2017 | Automation in Construction 82 (2017) 75–83 | State of the art methodologies | methodology | multimaterial optimization, building construction, energy efficiency AC | ||||
| 2019 | 156 | Efficient design optimization of variable-density cellular structures for additive manufacturing: Theory and experimental validation | Lin Cheng and Pu Zhang and Emre Biyikli and Jiaxi Bai and Joshua Robbins and Albert To | 2017 | Rapid Prototyping Journal , 23 ( 4 ) pp. 660 - 677 . | State of the art methodologies | methodology | Function (mechanics), Multiscale optimization (topological and lattice) | ||||
| 2019 | 157 | Grain-based Support Architecture Design for Additive Manufacturing | Md Ahasan Habib and Bashir Khoda | 2017 | Procedia Manufacturing , 10 pp. 876 - 886 | State of the art methodologies | methodology | function (manufacturing), optimization (support) | ||||
| 2019 | 158 ( 353) | Design optimization and validation of high-performance heat exchangers using approximation assisted optimization and additive manufacturing | Daniel Bacellar and Vikrant Aute and Zhiwei Huang and Reinhard Radermacher | 2017 | Science and Technology for the Built Environment , pp. 1 - 16 . | State of the art methodologies | methodology | Function (thermal and fluids), optimization (shape) | ||||
| 2019 | 159 | Design for additive manufacturing of porous structures using stochastic point-cloud: a pragmatic approach | A. M. M. Sharif Ullah | 2017 | Computer-Aided Design and Applications , Volume 15, Number 1 pp. 1 - 9 . | State of the art methodologies | methodology | Function (stochastic forms) | ||||
| 2019 | 160 | Design for additive bio-manufacturing: From patient-specific medical devices to rationally designed meta-biomaterials | Amir Zadpoor | 2017 | International Journal of Molecular Sciences 18(8),1607 | State of the art methodologies | methodology | Function (medical), state of the art, personalization, meta materials and bio materials. | ||||
| 2019 | 161 ( de 119) | Design methodology for porous composites with tunable thermal expansion produced by multi-material topology optimization and additive manufacturing | Takezawa, A., Kobashi, M. | 2017 | Composites Part B: Engineering 131, pp. 21-29 | State of the art methodologies | methodology | Function (expandable thermocontrollable form), optimization (topological) | ||||
| 2019 | 162 | Algorithm-driven design of fracture resistant composite materials realized through additive manufacturing | Grace X. Gu and Susan Wettermark and Markus J. Buehler | 2017 | Additive Manufacturing 17, pp. 47-54 | State of the art methodologies | methodology | Function (mechanics), multimaterial optimization. | ||||
| 2019 | 163 | User-centered design for additive manufacturing as a customization strategy | Ko, H., Enea, S., Chua, Z.Y., Moon, S.K., Otto, K.N. | 2016 | Proceedings of the International Conference on Progress in Additive Manufacturing , Part F129095 pp. 234 - 239 . | State of the art methodologies | methodology | Function (customization) | ||||
| 2019 | 164 | Customization design knowledge representation to support additive manufacturing | Ko, H., Moon, S.K., Otto, K | 2016 | Proceedings of the International Conference on Progress in Additive Manufacturing , Part F129095 pp. 13 - 18 . | State of the art methodologies | methodology | Function (customization) | ||||
| 2019 | 165 ( 141) | Design for Additive Manufacturing, to produce assembled products, by SLS | Bâlc, N., Vilǎu, C. | 2017 | MATEC Web of Conferences , 121 , art. no. 04002 | State of the art methodologies | methodology | Function (assembly) | ||||
| 2019 | 166 | The design for additive manufacturing worksheet | Booth, J.W., Alperovich, J., Chawla, P., Ma, J., Reid, T.N., Ramani, K. | 2017 | Journal of Mechanical Design, Transactions of the ASME , 139 ( 10 ) , art. no. 100904 | State of the art methodologies | methodology | design rules | ||||
| 2019 | 167 | Structural and mechanical characterization of custom design cranial implant created using additive manufacturing | Moiduddin, K., Darwish, S., Al-Ahmari, A., ElWatidy, S., Mohammad, A., Ameen, W. | 2017 | Electronic Journal of Biotechnology 29, pp. 22-31 | State of the art methodologies | methodology | Function (mechanics, customization, medicine) | ||||
| 2019 | 168 ( 123) | Cloud-based Design and Additive Manufacturing of Custom Orthoses | Shih, A., Park, D.W., Yang, Y.-Y., Chisena, R., Wu, D. | 2017 | Procedia CIRP 63, pp. 156-160 | State of the art methodologies | methodology | function/customization/medicine/innovation | ||||
| 2019 | 169 ( 122) | Systematic Biomimetic Part Design for Additive Manufacturing | Kamps, T., Gralow, M., Schlick, G., Reinhart, G. | 2017 | Procedia CIRP , 65 pp. 259 - 266 | State of the art methodologies | methodology | Function (resistance and weight) / optimization, innovation | ||||
| 2019 | 170 ( 140) | Design and Performance Assessment of Innovative Eco-Efficient Support Structures for Additive Manufacturing by Photopolymerization | Díaz Lantada, A., de Blas Romero, A., Sánchez Isasi, Á., Garrido Bellido, D. | 2017 | Journal of Industrial Ecology 21, pp. S179-S190 | State of the art methodologies | methodology | Function (mechanics and weight, ecological)/Optimization/innovation | ||||
| 2019 | 171 | Design and additive manufacturing of 3D phononic band gap structures based on gradient based optimization | Wormser, M., Wein, F., Stingl, M., Körner, C. | 2017 | Materials 10(10),1125 | State of the art methodologies | methodology | function (sound), Optimization (topological and lattice) | ||||
| 2019 | 172 | An approach to implement design for additive manufacturing in engineering studies | Lippert, B., Leuteritz, G., Lachmayer, R. | 2017 | Proceedings of the International Conference on Engineering Design, ICED, 5(DS87-5), pp. 51-60. | State of the art methodologies | methodology | education, DWX | ||||
| 2019 | 173 | Design heuristics for additive manufacturing | Blösch-Paidosh, A., Shea, K. | 2017 | Proceedings of the International Conference on Engineering Design, ICED 5(DS87-5), pp. 91-100 | State of the art methodologies | methodology | Design rule, conceptual design, state of the art (it is a research article, but it has many references) | ||||
| 2019 | 174 | The need for effective design guides in additive manufacturing | Seepersad, C.C., Allison, J., Sharpe, C. | 2017 | Proceedings of the International Conference on Engineering Design, ICED 5(DS87-5), pp. 309-316 | State of the art methodologies | methodology | design rule, conceptual design | ||||
| 2019 | 175 | A methodical approach to support ideation for additive manufacturing in design education | Watschke, H., Bavendiek, A.-K., Giannakos, A., Vietor, T. | 2017 | Proceedings of the International Conference on Engineering Design, ICED 5(DS87-5), pp. 41-50 | State of the art methodologies | methodology | design rule, conceptual design | ||||
| 2019 | 176 ( 126) | Physical Rigging for Physical Models and Posable Joint Designs Based on Additive Manufacturing Technology | Li, Y., Chen, Y. | 2017 | Procedia Manufacturing 11, pp. 2235-2242 | State of the art methodologies | methodology | Function (mechanics)/assembly | ||||
| 2019 | 177 ( 308) | Computational design and additive manufacturing of periodic conformal metasurfaces by synthesizing topology optimization with conformal mapping | Vogiatzis, P., Ma, M., Chen, S., Gu, X.D. | 2018 | Computer Methods in Applied Mechanics and Engineering 328, pp. 477-497 | State of the art methodologies | methodology | Functional (mechanical), optimization (topological, lattice) | ||||
| 2019 | 178 ( 133) | Which material design is possible under additive manufacturing: A fuzzy approach | Zapata, Francisco and Kosheleva, Olga and Kreinovich, Vladik | 2017 | 2017 Joint 17th World Congress of International Fuzzy Systems Association and 9th International Conference on Soft Computing and Intelligent Systems (IFSA-SCIS) | State of the art methodologies | methodology | Material selection, optimization (fuzzy logic) | ||||
| 2019 | 179 | Development of Automotive FlexBody Chassis Structure in Conceptual Design Phase using Additive Manufacturing | Kumar Dama, K., Kumar Malyala, S., Suresh Babu, V., Rao, R.N., Shaik, I.J. | 2017 | Materials Today: Proceedings, 4(9), pp. 9919-9923. | Functional (mechanical, automotive), conceptual design. | ||||||
| 2019 | 180 ( 131) | Design for additive manufacturing of customized cast with porous shell structures | Lim, Y.-E., Kim, N.-H., Choi, H.-J., Park, K. | 2017 | Journal of Mechanical Science and Technology, 31(11), pp. 5477-5483. | functional (customized, medical), optimization (porous) | ||||||
| 2019 | 181 | Additive manufacturing for RF microwave devices: Design, performances and treatments improvement evaluation | Talom, F.T., Turpault, S. | 2017 | Proceedings of the 2017 19th International Conference on Electromagnetics in Advanced Applications, ICEAA 2017, art. no. 8065560, pp. 1473-1476. | State of the art methodologies | methodology | Functional (electronics), multiprocess (electroplating) | ||||
| 2019 | 182 | Practical considerations in the design of monoblock TM dielectric resonator filters with additive manufacturing | Carceller, C., Gentili, F., Reichartzeder, D., Bösch, W., Schwentenwein, M | 2017 | Proceedings of the 2017 19th International Conference on Electromagnetics in Advanced Applications, ICEAA 2017, art. no. 8065251, pp. 364-367 | State of the art methodologies | methodology | Functional (electronics), multiprocessing | ||||
| 2019 | 183 | Design considerations for additive manufacturing of feed channel spacers for spiral wound membrane modules | An, J., Tan, W.S., Chua, C.K., Chong, T.H., Fane, A.G. | 2017 | Challenges for Technology Innovation: An Agenda for the Future - Proceedings of the International Conference on Sustainable Smart Manufacturing, S2M 2016, pp. 211-216. | State of the art methodologies | methodology | Functional (fluids) | ||||
| 2019 | 184 ( 147) | A hybrid machine learning approach for additive manufacturing design feature recommendation | Yao, X., Moon, S.K., Bi, G. | 2017 | Rapid Prototyping Journal, 23(6), pp. 983-997. | optimization (machine learning) | ||||||
| 2019 | 185 ( 146) | A method for modularity in design rules for additive manufacturing | Jee, H., Witherell, P. | 2017 | Rapid Prototyping Journal, 23(6), pp. 1107-1118. | Function (assembly) | ||||||
| 2019 | 186 ( 136) | New to Power Equipment Design Approaches with Additive Manufacturing prospects | Belova, O.V., Vulf, M.D. | 2017 | Journal of Physics: Conference Series, 891(1), art. no. 012211. | turbomachinery, thermofluids | ||||||
| 2019 | 187 | Study, design and prototyping of arm splint with additive manufacturing process | Blaya, F., D'amato, R., Pedro, P.S., (...), Lopez-Silva, J., Lagándara, J.G. | 2017 | ACM International Conference Proceeding Series, Part F132203, art. no. 57. | State of the art methodologies | methodology | function (medical) | ||||
| 2019 | 188 | Low weight additive manufacturing FBG accelerometer: Design, characterization and testing | Gutiérrez, N., Galvín, P., Lasagni, F | 2018 | Measurement: Journal of the International Measurement Confederation, 117, pp. 295-303. | State of the art methodologies | methodology | Function (electronics, instrumentation) | ||||
| 2019 | 189 | Mesoscale design of heterogeneous material systems in multi-material additive manufacturing | Garcia, D., Jones, M.E., Zhu, Y., Yu, H.Z. | 2018 | Journal of Materials Research, 33(1), pp. 58-67. | State of the art methodologies | methodology | Optimization (multiscale, multimaterial) | ||||
| 2019 | 190 | The scope of additive manufacturing in cryogenics, component design, and applications | Stautner, W., Vanapalli, S., Weiss, K.-P., (...), Budesheim, E., Ricci, J. | 2017 | IOP Conference Series: Materials Science and Engineering, 278(1), art. no. 012134 | State of the art methodologies | methodology | termofluidos, estado del arte, criogenia translated to English is thermofluids, state of the art, cryogenics. | ||||
| 2019 | 191 | Additive Design and Manufacturing of Jet Engine Parts | Han, P. | 2017 | Engineering, 3(5), pp. 648-652. | State of the art methodologies | methodology | fluid terms, design rules | ||||
| 2019 | 192 | Design and additive manufacturing of lower limb prosthetic socket | Vitali, A., Regazzoni, D., Rizzi, C., Colombo, G | 2017 | ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE), 11. | State of the art methodologies | methodology | function (doctor) | ||||
| 2019 | 193 | Design of novel materials for additive manufacturing - Isotropic microstructure and high defect tolerance | Günther, J., Brenne, F., Droste, M., (...), Biermann, H., Niendorf, T. | 2018 | Scientific Reports, 8(1), art. no. 1298. | State of the art methodologies | methodology | MECHANICS AND TOLERANCES | ||||
| 2019 | 194 | Design and fabrication of integrated micro/macrostructure for 3D functional gradient systems based on additive manufacturing | Yin, M., Xie, L., Jiang, W., Yin, G | 2018 | Optics Communications, 414, pp. 195-201. | State of the art methodologies | methodology | electronics, multiscale optimization | ||||
| 2019 | 195 ( 307) | Design of graded lattice structure with optimized mesostructures for additive manufacturing | Wang, Y., Zhang, L., Daynes, S., (...), Feih, S., Wang, M.Y. | 2018 | Materials and Design, 142, pp. 114-123. | State of the art methodologies | methodology | optimization (multiple scale, topological, lattice) | ||||
| 2019 | 196 | Design and additive manufacturing of multi-Permeability magnetic cores | Liu, L., Ding, C., Lu, S., (...), Ngo, K.D.T., Lu, G.-Q. | 2017 | 2017 IEEE Energy Conversion Congress and Exposition, ECCE 2017, 2017-January, art. no. 8095878, pp. 881-886. | State of the art methodologies | methodology | Sure, I can help you with that. Here is the translation of electrica into English: electric I have removed the quotation and double quotation marks from the translated value as requested. | ||||
| 2019 | 197 | Design approach for additive manufacturing employing Constructal Theory for point-to-circle flows | Kamps, T., Biedermann, M., Seidel, C., Reinhart, G. | 2018 | Additive Manufacturing, 20, pp. 111-118. | State of the art methodologies | methodology | Sure, I can help you with that. Here is the translation of electrica into English: electric I have removed the quotation and double quotation marks from the translated value as requested. | ||||
| 2019 | 198 (es mas para otros) | Mechatronic design for an extrusion-based additive manufacturing machine | Giberti, H., Sbaglia, L., Silvestri, M. | 2017 | Machines, 5(4), art. no. 29. | State of the art methodologies | another | mechatronics | ||||
| 2019 | 199 ( 152) | Manufactured chemistry: Rethinking unit operation design in the age of additive manufacturing | Stark, A.K. | 2018 | AIChE Journal. Article in Press | State of the art methodologies | ||||||
| 2019 | 200 | Lifecycle design and management of additive manufacturing technologies | Müller, J.R., Panarotto, M., Malmqvist, J., Isaksson, O. | 2018 | Procedia Manufacturing, 19, pp. 135-142. | State of the art methodologies | methodology | management, life cycle | ||||
| 2019 | 201 | Aiming for modeling-assisted tailored designs for additive manufacturing | Gunasegaram, D.R., Murphy, A.B., Cummins, S.J., (...), Nguyen, V., Feng, Y. | 2017 | Minerals, Metals and Materials Series, Part F6, pp. 91-102. | State of the art methodologies | methodology | Function (mechanics), optimization (mesoscale: homogeneous (lattice)) | ||||
| 2019 | 202 (en japones) | Design and development of intervertebral fusion cage with novel concept by metal powder-based additive manufacturing | Takahashi, H., Nakashima, Y., Ito, M., Ishimoto, T., Nakano, T. | 2018 | Funtai Oyobi Fummatsu Yakin/Journal of the Japan Society of Powder and Powder Metallurgy, 65(2), pp. 132-134. | State of the art methodologies | methodology | |||||
| 2019 | 203 | A Knowledge Management System to Support Design for Additive Manufacturing Using Bayesian Networks | Wang, Y., Blache, R., Zheng, P., Xu, X. | 2018 | Journal of Mechanical Design, Transactions of the ASME, 140(5), art. no. 051701. | State of the art methodologies | methodology | Database, process and material selection, state of the art (it is a research article but has many references), design rules (fused deposition) | ||||
| 2019 | 204 | Investigation of design for additive manufacturing in professional design practice | Pradel, P., Zhu, Z., Bibb, R., Moultrie, J. | 2018 | Journal of Engineering Design, pp. 1-36. | State of the art methodologies | methodology | DFAM, design rules, STATE OF THE ART (research article but with many references) | ||||
| 2019 | 205 | Toward integrated design of additive manufacturing through a process development model and multi-objective optimization | Asadollahi-Yazdi, E., Gardan, J., Lafon, P. | 2018 | International Journal of Advanced Manufacturing Technology, pp. 1-20. | State of the art methodologies | methodology | Optimization (multi-objective), STATE OF THE ART (research article but with many references) | ||||
| 2019 | 206 | Design of an Orthopedic Product by Using Additive Manufacturing Technology: The Arm Splint | Blaya, F., Pedro, P.S., Silva, J.L., (...), Heras, E.S., Juanes, J.A. | 2018 | Journal of Medical Systems, 42(3), art. no. 54. | State of the art methodologies | methodology | medical, personalization | ||||
| 2019 | 207 | Design optimization and additive manufacturing of nodes in gridshell structures | Seifi, H., Rezaee Javan, A., Xu, S., Zhao, Y., Xie, Y.M. | 2018 | Engineering Structures, 160, pp. 161-170. | State of the art methodologies | methodology | optimization, thermofluids, topological | ||||
| 2019 | 208 | Dynamic supply chain design and operations plan for connected smart factories with additive manufacturing | Do Chung, B., Kim, S.I., Lee, J.S. | 2018 | Applied Sciences (Switzerland), 8(4), art. no. 583. | State of the art methodologies | methodology | Supply chain | ||||
| 2019 | 209 | Topology optimization as an innovative design method for additive manufacturing | Nguyen, D.S., Vignat, F. | 2018 | IEEE International Conference on Industrial Engineering and Engineering Management, 2017-December, pp. 304-308. | State of the art methodologies | methodology | optimization (topological) | ||||
| 2019 | 210 | Design and manufacturing of high-performance prostheses with additive manufacturing and fiber-reinforced polymers | Türk, D.-A., Einarsson, H., Lecomte, C., Meboldt, M. | 2018 | Production Engineering, pp. 1-11. | State of the art methodologies | methodology | medical, mechanics, customization | ||||
| 2019 | 211 | A Realization Method for Transforming a Topology Optimization Design into Additive Manufacturing Structures | Liu, S., Li, Q., Liu, J., Chen, W., Zhang, Y. | 2018 | Engineering. | State of the art methodologies | methodology | Optimization (topological) | ||||
| 2019 | 212 | Novel topological design of 3D Kagome structure for additive manufacturing | Wang, R., Shang, J., Li, X., Wang, Z., Luo, Z. | 2018 | Rapid Prototyping Journal, 24(2), pp. 261-269. | State of the art methodologies | methodology | Optimization (topological, lattice) | ||||
| 2019 | 213 | A Study of Design Fixation Related to Additive Manufacturing | Abdelall, E.S., Frank, M.C., Stone, R.T. | 2018 | Journal of Mechanical Design, Transactions of the ASME, 140(4), art. no. 041702. | State of the art methodologies | methodology | ensemble, state of the art (research article but many references) Please note that the translation may vary depending on the context and specific meaning of the words. | ||||
| 2019 | 214 | A multi-material part design framework in additive manufacturing | Yao, X., Moon, S.K., Bi, G., Wei, J. | 2018 | International Journal of Advanced Manufacturing Technology, pp. 1-9. | State of the art methodologies | methodology | multimaterial | ||||
| 2019 | 215 | Traditional or Additive Manufacturing? Assessing Component Design Options through Lifecycle Cost Analysis | Westerweel, B., Basten, R.J.I., van Houtum, G.-J. | 2018 | European Journal of Operational Research. | State of the art methodologies | methodology | cost, life cycle | ||||
| 2019 | 216 | Part decomposition and assembly-based (Re) design for additive manufacturing: A review | Oh, Y., Zhou, C., Behdad, S. | 2018 | Additive Manufacturing, 22, pp. 230-242. | State of the art methodologies | methodology | ensemble, state of the art | ||||
| 2019 | 217 | The Role of re-design for Additive Manufacturing on the Process Environmental Performance | Priarone, P.C., Ingarao, G., Lunetto, V., Di Lorenzo, R., Settineri, L. | 2018 | Procedia CIRP, 69, pp. 124-129. | State of the art methodologies | methodology | environment, surroundings | ||||
| 2019 | 218 | Topology optimization and laser additive manufacturing in design process of efficiency lightweight aerospace parts | Fetisov, K.V., Maksimov, P.V. | 2018 | Journal of Physics: Conference Series, 1015(5), art. no. 052006, p. 8DUMMY. | State of the art methodologies | methodology | topological optimization, aerospace mechanics | ||||
| 2019 | 219 | Understanding the scope for a product design education discourse on additive manufacturing | Loy, J. | 2018 | Archives of Design Research, 31(2), pp. 15-23. | State of the art methodologies | methodology | DWAM, state of the art | ||||
| 2019 | 220 | Additive manufacturing-driven mold design for castings | Kang, J., Shangguan, H., Deng, C., (...), Zhang, X., Huang, T. | 2018 | Additive Manufacturing, 22, pp. 472-478. | State of the art methodologies | methodology | Design of Casting Molds, Thermofluids | ||||
| 2019 | 221 | A framework for mapping design for additive manufacturing knowledge for industrial and product design | Pradel, P., Zhu, Z., Bibb, R., Moultrie, J. | 2018 | Journal of Engineering Design, pp. 1-36. | State of the art methodologies | methodology | DFAM, DFX mod AM, state of the art (it is a research article but has many references) | ||||
| 2019 | 222 | Structural topology optimization for generative design of personalized aneurysm implants: Design, additive manufacturing, and experimental validation | Jiang, L., Chen, S., Sadasivan, C., Jiao, X. | 2017 | 2017 IEEE Healthcare Innovations and Point of Care Technologies, HI-POCT 2017, 2017-December, pp. 9-13. | State of the art methodologies | methodology | Topological optimization, medical | ||||
| 2019 | 223 | Numerical comparison of lattice unit cell designs for medical implants by additive manufacturing | du Plessis, A., Yadroitsava, I., Yadroitsev, I., le Roux, S., Blaine, D. | 2018 | Virtual and Physical Prototyping, pp. 1-16. | State of the art methodologies | methodology | Optimization (lattice), medical | ||||
| 2019 | 224 | Breathable tissue engineering scaffolds: An efficient design-optimization by additive manufacturing | Touri, M., Moztarzadeh, F., Osman, N.A.A., Dehghan, M.M., Mozafari, M. | 2018 | Materials Today: Proceedings, 5(7), pp. 15813-15820. | State of the art methodologies | methodology | Optimization (lattice), medical | ||||
| 2019 | 225 | Role of CT and MRI in the design and development of orthopaedic model using additive manufacturing | Haleem, A., Javaid, M. | 2018 | Journal of Clinical Orthopaedics and Trauma. | State of the art methodologies | methodology | medical, state of the art | ||||
| 2019 | 226 | Optimal design and modeling of gyroid-based functionally graded cellular structures for additive manufacturing | Li, D., Liao, W., Dai, N., (...), Tang, Y., Xie, Y.M. | 2018 | CAD Computer Aided Design, 104, pp. 87-99. | State of the art methodologies | methodology | optimization (lattice, topological), mechanics | ||||
| 2019 | 227 | Invited review article: Metal-additive manufacturing—Modeling strategies for application-optimized designs | Bandyopadhyay, A., Traxel, K.D. | 2018 | Additive Manufacturing, 22, pp. 758-774. | State of the art methodologies | methodology | Optimization (topological, lattice), multimaterial function (mechanics), state of the art. | ||||
| 2019 | 228 | Design By Additive Manufacturing: an application in aeronautics and defence | Segonds, F. | 2018 | Virtual and Physical Prototyping, pp. 1-9. | State of the art methodologies | methodology | mechanics, aerospace, innovation | ||||
| 2019 | 229 | Reliability centered additive manufacturing computational design framework | Harris, P., Laskowski, B., Reutzel, E., Earthman, J.C., Hess, A.J. | 2018 | IEEE Aerospace Conference Proceedings, 2018-March, pp. 1-10. | State of the art methodologies | methodology | aerospace, system or database | ||||
| 2019 | 230 | Application of additive manufacturing in design & manufacturing engineering education | Keaveney, S.G., Dowling, D.P. | 2018 | 2018 2nd International Symposium on Small-Scale Intelligent Manufacturing Systems, SIMS 2018, 2018-January, pp. 1-6. | DWAM, educational | ||||||
| 2019 | 231 | Design optimization of heat sink using additive manufacturing | Tateishi, Y., Parque, V., Miyashita, T., (...), Kato, R., Ikeda, Y. | 2017 | 2017 IEEE CPMT Symposium Japan, ICSJ 2017, 2017-January, pp. 91-94. | State of the art methodologies | methodology | termofluids | ||||
| 2019 | 232 | An ergonomic customized-tool handle design for precision tools using additive manufacturing: A case study | González, A.G., Salgado, D.R., Moruno, L.G., Ríos, A.S. | 2018 | Applied Sciences (Switzerland), 8(7), art. no. 1200. | State of the art methodologies | methodology | customization, tooling | ||||
| 2019 | 233 | A semi-automated virtual workflow solution for the design and production of intraoral molding plates using additive manufacturing: the first clinical results of a pilot-study | Grill, F.D., Ritschl, L.M., Bauer, F.X., (...), Wolff, K.-D., Loeffelbein, D.J. | 2018 | Scientific Reports, 8(1), art. no. 11845. | State of the art methodologies | methodology | medical, personalization | ||||
| 2019 | 234 | A Conceptual Design and Modeling Framework for Integrated Additive Manufacturing | Mokhtarian, H., Coatanéa, E., Paris, H., (...), Vihinen, J., Ellman, A. | 2018 | Journal of Mechanical Design, Transactions of the ASME, 140(8), art. no. 081101. | State of the art methodologies | methodology | Conceptual design, FDM | ||||
| 2019 | 235 | Additive manufacturing for industrial benchmarking: An application to vehicle's under-hood design | Naddeo, A., Cappetti, N. | 2018 | ARPN Journal of Engineering and Applied Sciences, 13(14), pp. 4292-4299. | State of the art methodologies | methodology | Benchmarking (conceptual design) | ||||
| 2019 | 236 | Feature-Based Methodology for Design of Geometric Benchmark Test Artifacts for Additive Manufacturing Processes | Rupal, B.S., Ahmad, R., Qureshi, A.J. | 2018 | Procedia CIRP, 70, pp. 84-89. | State of the art methodologies | methodology | DFAM, tolerances, assembly, adjustments | ||||
| 2019 | 237 ( 334) | Integrated design-oriented framework for Resource Selection in Additive Manufacturing | Uz Zaman, U.K., Rivette, M., Siadat, A., Baqai, A.A. | 2018 | Procedia CIRP, 70, pp. 96-101. | State of the art methodologies | methodology | DFAM, material selection and processes, cost, function, sustainability | ||||
| 2019 | 238 | Planning, Evaluation and Optimization of Product Design and Manufacturing Technology Chains for New Product and Production Technologies on the Example of Additive Manufacturing | Jacob, A., Windhuber, K., Ranke, D., Lanza, G. | 2018 | Procedia CIRP, 70, pp. 108-113. | State of the art methodologies | methodology | DFAM, material selection and processes (hybrid) | ||||
| 2019 | 239 | Design of a scaffold parameter selection system with additive manufacturing for a biomedical cell culture | Rabionet, M., Polonio, E., Guerra, A.J., (...), Puig, T., Ciurana, J. | 2018 | Materials, 11(8), art. no. 1427. | State of the art methodologies | methodology | medicine, cell growth structure printing, FDM | ||||
| 2019 | 240 | Design & manufacture of a high-performance bicycle crank by Additive Manufacturing | McEwen, I., Cooper, D.E., Warnett, J., (...), Williams, M.A., Gibbons, G.J. | 2018 | Applied Sciences (Switzerland), 8(8), art. no. 1360. | State of the art methodologies | methodology | mechanics, weight | ||||
| 2019 | 241 | Structural design and mechanical response of gradient porous Ti-6Al-4V fabricated by electron beam additive manufacturing | Wu, Y.C., Kuo, C.N., Shie, M.Y., (...), Chen, S.Y., Huang, J.C. | 2018 | Materials and Design, 158, pp. 256-265. | State of the art methodologies | methodology | mechanics | ||||
| 2019 | 242 | Additive Manufacturing as a Method to Design and Optimize Bioinspired Structures | Velasco-Hogan, A., Xu, J., Meyers, M.A. | 2018 | Advanced Materials. | State of the art methodologies | methodology | State of the art, multiscale, lattice | ||||
| 2019 | 243 | Bioinspired hierarchical composite design using machine learning: Simulation, additive manufacturing, and experiment | Gu, G.X., Chen, C.-T., Richmond, D.J., Buehler, M.J. | 2018 | Materials Horizons, 5(5), pp. 939-945. | State of the art methodologies | methodology | multimaterial, latice, machine learnig | ||||
| 2019 | 244 | Additive Manufacturing Handbook. | Badiru, A. (Ed.), Valencia, V. (Ed.), Liu, D. (Ed.). | 2017 | Boca Raton: CRC Press, | State of the art methodologies | manufacturing Please note that I have removed the quotation and double quotation marks from the translated value. | state of the art. | ||||
| 2019 | 245 | Additive Manufacturing with Bioinspired Sustainable Product Design: A Conceptual Model | Zhang, H., Nagel, J.K., Al-Qas, A., Gibbons, E., Lee, J.J.-Y. | 2018 | Procedia Manufacturing, 26, pp. 880-891. | State of the art methodologies | methodology | multiescala, latice, conceptual design | ||||
| 2019 | 246 | Design Right Once for Additive Manufacturing | Tsakiris, A., Salpistis, C., Mihailidis, A. | 2018 | MATEC Web of Conferences, 188, art. | State of the art methodologies | methodology | innovation, mechanics, topological optimization, conceptual design | ||||
| 2019 | 247 | Manufacturing elements to support design for additive manufacturing | Rosen, D.W. | 2018 | Proceedings of the International Conference on Progress in Additive Manufacturing, 2018-May, pp. 309-314. | State of the art methodologies | methodology | DFAM, database | ||||
| 2019 | 248 | Linking part design to process planning by design for additive manufacturing ontology | Kim, S., Rosen, D.W., Witherell, P., Ko, H. | 2018 | Proceedings of the International Conference on Progress in Additive Manufacturing, 2018-May, pp. 303-308. | State of the art methodologies | methodology | DFAM, database | ||||
| 2019 | 249 | Fly without borders with additive manufacturing: A microscale tilt-rotor tricopter design | Lee, Y.W., Mehndiratta, M., Kayacan, E. | 2018 | Proceedings of the International Conference on Progress in Additive Manufacturing, 2018-May, pp. 256-261. | State of the art methodologies | methodology | DFAM | ||||
| 2019 | 250 | Integrating parametric design with robotic additive manufacturing for 3D clay printing: An experimental study | Kontovourkis, O., Tryfonos, G. | 2018 | ISARC 2018 - 35th International Symposium on Automation and Robotics in Construction and International AEC/FM Hackathon: The Future of Building Things. | State of the art methodologies | manufacturing Please note that I have removed the quotation and double quotation marks from the translated value. | empirical, design rule, construction | ||||
| 2019 | 251 | A design framework for additive manufacturing through the synergistic use of axiomatic design theory and TRIZ | Renjith, S.C., Okudan Kremer, G.E., Park, K. | 2018 | IISE Annual Conference and Expo 2018, pp. 551-556. | State of the art methodologies | methodology | Modified DFX for AM | ||||
| 2019 | 252 | Design and strengthening mechanisms in hierarchical architected materials processed using additive manufacturing | Sha, Y., Jiani, L., Haoyu, C., Ritchie, R.O., Jun, X. | 2018 | International Journal of Mechanical Sciences, 149, pp. 150-163. | State of the art methodologies | methodology | lattice, mechanics | ||||
| 2019 | 253 | Design, finite element analysis (FEA), and fabrication of custom titanium alloy cranial implant using electron beam melting additive manufacturing | Ameen, W., Al-Ahmari, A., Mohammed, M.K., (...), Umer, U., Moiduddin, K. | 2018 | Advances in Production Engineering And Management, 13(3), pp. 267-278. | State of the art methodologies | methodology | medical, mechanical | ||||
| 2019 | 254 | Design of a 4 degrees of freedom decoupled monolithic compliant alignment mechanism for additive manufacturing | Van Hoek, N., Van Der Wijk, V., Herder, J., Oosterhuis, G. | 2018 | European Society for Precision Engineering and Nanotechnology, Conference Proceedings - 18th International Conference and Exhibition, EUSPEN 2018, pp. 297-298. | State of the art methodologies | methodology | mechanism, assembly | ||||
| 2019 | 255 | A new design for an extensive benchmarking of additive manufacturing machines | Moshiri, M., Tosello, G., Mohanty, S. | 2018 | European Society for Precision Engineering and Nanotechnology, Conference Proceedings - 18th International Conference and Exhibition, EUSPEN 2018, pp. 261-262. | State of the art methodologies | methodology | benchmarking | ||||
| 2019 | 256 | Trivariate spline representations for computer aided design and additive manufacturing | Dokken, T., Skytt, V., Barrowclough, O. | 2018 | Computers and Mathematics with Applications. | State of the art methodologies | methodology | |||||
| 2019 | 257 | Evaluating design heuristics for additive manufacturing as an explorative workshop method | Lindwall, A., Törlind, P. | 2018 | Proceedings of International Design Conference, DESIGN, 3, pp. 1221-1232. | State of the art methodologies | methodology | Design rules, heuristics, state of the art | ||||
| 2019 | 258 | Impact on design when introducing additive manufacturing in space applications | Borgue, O., Panarotto, M., Isaksson, O. | 2018 | Proceedings of International Design Conference, DESIGN, 3, pp. 997-1008. | State of the art methodologies | methodology | Advantages/limitations, function (mechanics, weight), state of the art. | ||||
| 2019 | 259 | Using the potentials of additive manufacturing by a systematic linkage of the manufacturing process to product design | Würtenberger, J., Reichwein, J., Kirchner, E. | 2018 | Proceedings of International Design Conference, DESIGN, 3, pp. 1465-1476. | State of the art methodologies | methodology | advantages | ||||
| 2019 | 260 | Additive manufacturing of elastomeric foam with cell unit design for broadening compressive stress plateau | Zhu, X., Chen, Y., Liu, Y., (...), Liu, T., Yang, J. | 2018 | Rapid Prototyping Journal. | State of the art methodologies | methodology | lattice, mechanics | ||||
| 2019 | 261 | Re-design and re-manufacturing of discontinued spare parts implementing additive manufacturing in the military field | Montero, J., Paetzold, K., Bleckmann, M., Holtmannspoetter, J. | 2018 | Proceedings of International Design Conference, DESIGN, 3, pp. 1269-1278. | State of the art methodologies | methodology | redesign and remanufacturing | ||||
| 2019 | 262 | Design for additive manufacturing: Mapping of product functions | Valjak, F., Bojčetić, N., Lukić, M. | 2018 | Proceedings of International Design Conference, DESIGN, 3, pp. 1369-1380. | State of the art methodologies | methodology | ontological, conceptual design, advantages | ||||
| 2019 | 263 | Optimization design of nonuniform cellular structures for additive manufacturing | Han, Y., Lu, W.F. | 2018 | ASME 2018 13th International Manufacturing Science and Engineering Conference, MSEC 2018, 1. | State of the art methodologies | methodology | lattice, mechanics | ||||
| 2019 | 264 | Design of high-manganese steels for additive manufacturing applications with energy-absorption functionality | Kies, F., Köhnen, P., Wilms, M.B., (...), Schleifenbaum, J.H., Haase, C. | 2018 | Materials and Design, 160, pp. 1250-1264. | State of the art methodologies | methodology | mechanics, lattice | ||||
| 2019 | 265 | Powder bed fusion metrology for additive manufacturing design guidance | Allison, J., Sharpe, C., Seepersad, C.C. | 2019 | Additive Manufacturing, 25, pp. 239-251. | State of the art methodologies | methodology | Design rule, tolerance | ||||
| 2019 | 266 ( 237) | Digital design and nonlinear simulation for additive manufacturing | Weeger, O., Boddeti, N., Yeung, S.-K., Kaijima, S., Dunn, M.L. | 2019 | Additive Manufacturing, 25, pp. 39-49. | State of the art methodologies | methodology | lattice, mechanics | ||||
| 2019 | 267 | Study on Nature-inspired Fractal Design-based Flexible Counter Electrodes for Dye-Sensitized Solar Cells Fabricated using Additive Manufacturing | James, S., Contractor, R. | 2018 | Scientific Reports, 8(1), art. no. 17032. | State of the art methodologies | methodology | Translated data: lattice, electric, electronics, multiprocessing | ||||
| 2019 | 268 | Computational design of nanostructural color for additive manufacturing | Auzinger, T., Heidrich, W., Bickel, B. | 2018 | ACM Transactions on Graphics, 37(4), art. no. 159. | State of the art methodologies | methodology | optics, multiscale, optimization | ||||
| 2019 | 269 | Design and experimental testing of a Mini Channel Heat Exchanger made in Additive Manufacturing | Cardone, M., Gargiulo, B. | 2018 | Energy Procedia, 148, pp. 932-939. | State of the art methodologies | methodology | termofluids | ||||
| 2019 | 270 | Design for additive manufacturing inspired by TRIZ | Gross, J., Park, K., Okudan Kremer, G.E. | 2018 | Proceedings of the ASME Design Engineering Technical Conference, 4. | State of the art methodologies | methodology | Modified DFX for AM | ||||
| 2019 | 271 | Manufacturability constraint formulation for design under hybrid additive-subtractive manufacturing | Patterson, A.E., Allison, J.T. | 2018 | Proceedings of the ASME Design Engineering Technical Conference, 4. | State of the art methodologies | methodology | DFAM with subtractive (multiprocesses) | ||||
| 2019 | 272 | A novel approaches to components design additive manufacturing process | Orlov, A.V., Masaylo, D.V., Sufiiarov, V.S., (...), Polozov, I.A., Popovich, A.A. | 2018 | IOP Conference Series: Earth and Environmental Science, 194(2), art. no. 022026. | State of the art methodologies | methodology | topological optimization, mechanics | ||||
| 2019 | 273 | Design for additive manufacturing of conformal cooling channels using thermal-fluid topology optimization and application in injection molds | Wu, T., Tovar, A. | 2018 | Proceedings of the ASME Design Engineering Technical Conference, 2B-2018. | State of the art methodologies | methodology | termofluids, optimization (topological) | ||||
| 2019 | 274 | Function modelling and constraints replacement to support design for additive manufacturing of satellite components | Borgue, O., Muller, J., Panarotto, M., Isaksson, O. | 2018 | Proceedings of NordDesign: Design in the Era of Digitalization, NordDesign 2018. | State of the art methodologies | methodology | Advantages and restrictions | ||||
| 2019 | 275 | Integrating additive manufacturing and repair strategies of aeroengine components in the computational multidisciplinary engineering design process | Handawi, K.A., Lawand, L., Andersson, P., (...), Isaksson, O., Kokkolaras, M. | 2018 | Proceedings of NordDesign: Design in the Era of Digitalization, NordDesign 2018. | State of the art methodologies | methodology | Maintenance, mechanical resistance, life cycle, costs | ||||
| 2019 | 276 | Design for qualification: A process for developing additive manufacturing components for critical systems | Dordlofva, C., Törlind, P. | 2018 | Proceedings of NordDesign: Design in the Era of Digitalization, NordDesign 2018. | State of the art methodologies | methodology | DFX modified for AM, DFQ | ||||
| 2019 | 277 | Design for additive manufacturing (DfAM) methodologies: a proposal to foster the design of microwave waveguide components | François, M., Segonds, F., Rivette, M., Turpault, S., Peyre, P. | 2018 | Virtual and Physical Prototyping. | State of the art methodologies | methodology | DFAM | ||||
| 2019 | 278 | Enabling graduate students to design for additive manufacturing through teaching and experience transfer | Ferchow, J., Klahn, C., Meboldt, M. | 2018 | Proceedings of the 20th International Conference on Engineering and Product Design Education, E and PDE 2018. | State of the art methodologies | methodology | DWAM, educational | ||||
| 2019 | 279 | Joint Asymmetric Tolerance Design and Manufacturing Decision-Making for Additive Manufacturing Processes | Haghighi, A., Li, L. | 2018 | IEEE Transactions on Automation Science and Engineering. | State of the art methodologies | methodology | ensemble, tolerance | ||||
| 2019 | 280 ( 316) | Creativity and productivity in product design for additive manufacturing: Mechanisms and platform outcomes of remixing | Friesike, S., Flath, C.M., Wirth, M., Thiesse, F. | 2018 | Journal of Operations Management. | State of the art methodologies | methodology | innovation, creativity, virtual design | ||||
| 2019 | 281 | Integrating additive manufacturing in the design of aerospace components | Stolt, R., Heikkinen, T., Elgh, F. | 2018 | Advances in Transdisciplinary Engineering, 7, pp. 145-154. | State of the art methodologies | methodology | mechanics, weight | ||||
| 2019 | 282 | Thermal design, optimization and additive manufacturing of ceramic regular structures to maximize the radiative heat transfer | Pelanconi, M., Barbato, M., Zavattoni, S., Vignoles, G.L., Ortona, A. | 2019 | Materials and Design, 163, art. no. 107539. | State of the art methodologies | methodology | fluid term, lattice | ||||
| 2019 | 283 | A novel optimization design method of additive manufacturing oriented porous structures and experimental validation | Zhao, J., Zhang, M., Zhu, Y., (...), Wang, L., Hu, J. | 2019 | Materials and Design, 163, art. no. 107550. | State of the art methodologies | methodology | Optimization (topological), function (mechanics, weight) | ||||
| 2019 | 284 | Advanced design applied to an original multi-purpose ventilator achievable by additive manufacturing | Frizziero, L., Donnici, G., Dhaimini, K., Liverani, A., Caligiana, G. | 2018 | Applied Sciences (Switzerland), 8(12), art. no. 2635. | State of the art methodologies | methodology | Modified dfx for AM, optimization (multipurpose), thermofluids | ||||
| 2019 | 285 | Comparison of a transtibial socket design obtained by additive manufacturing and reverse engineering and a traditional model | Salamanca Jaimes, E., Prada Botiá, G.C., Rodrigues, P.H., (...), Campos Rubio, J.C., Volpini Lana, M.R. | 2018 | Journal of Physics: Conference Series, 1126(1), art. no. 012016. | State of the art methodologies | methodology | medicine | ||||
| 2019 | 286 | Design and manufacture of orthopedic corset using 3D digitization and additive manufacturing | Molnár, I., Morovič, L. | 2018 | IOP Conference Series: Materials Science and Engineering, 448(1), art. no. 012058. | State of the art methodologies | methodology | medicine | ||||
| 2019 | 287 | Design and prototyping by additive manufacturing of a functional splint for rehabilitation of Achilles tendon intrasubstance rupture | Haro, F.B., Lopez-Silva, J., Pedro, P.S., (...), Pedro, A.B.S., D'Amato, R. | 2018 | ACM International Conference Proceeding Series, pp. 433-439. | State of the art methodologies | methodology | medicine | ||||
| 2019 | 288 | TEAM: A tool for eco additive manufacturing to optimize environmental impact in early design stages | Floriane, L., Enrico, B., Frédéric, S., (...), Gianluca, D.A., Paolo, C. | 2018 | IFIP Advances in Information and Communication Technology, 540, pp. 736-746. | State of the art methodologies | methodology | environment | ||||
| 2019 | 289 | Design, development and characterization of linear, soft actuators via additive manufacturing | Costas, A., Davis, D.E., Niu, Y., (...), Garcia, J., Newell, B. | 2018 | ASME 2018 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, SMASIS 2018, 1. | State of the art methodologies | methodology | automation, robotics, multiprocessing | ||||
| 2019 | 290 | Multi-view feature modeling for design-for-additive manufacturing | Li, L., Liu, J., Ma, Y., Ahmad, R., Qureshi, A. | 2019 | Advanced Engineering Informatics, 39, pp. 144-156. | State of the art methodologies | methodology | Optimization (multi-objective, topological, lattice) | ||||
| 2019 | 291 | Hydraulic manifold design via additive manufacturing optimized with CFD and fluid-structure interaction simulations | Alshare, A.A., Calzone, F., Muzzupappa, M. | 2018 | Rapid Prototyping Journal. | State of the art methodologies | methodology | termofluids | ||||
| 2019 | 292 | Design for additive manufacturing: Benefits, trends and challenges | Durakovic, B. | 2018 | Periodicals of Engineering and Natural Sciences, 6(2), pp. 179-191. | State of the art methodologies | methodology | state of the art. | ||||
| 2019 | 293 | Design of Air Cooling Housing for Image Sensors Using Additive Manufacturing Technology | Kim, C., Hillstrom, A., Coronel, J., (...), Espalin, D., Wicker, R. | 2018 | 2018 International Conference on Information and Communication Technology Robotics, ICT-ROBOT 2018, art. no. 8549891. | State of the art methodologies | methodology | termofluids | ||||
| 2019 | 294 | Laser powder bed fusion (L-PBF) additive manufacturing: On the correlation between design choices and process sustainability | Priarone, P.C., Lunetto, V., Atzeni, E., Salmi, A. | 2018 | Procedia CIRP, 78, pp. 85-90. | State of the art methodologies | methodology | sustainability, environment | ||||
| 2019 | 295 | Homogenization driven design of lightweight structures for additive manufacturing | Savio, G., Curtarello, A., Rosso, S., Meneghello, R., Concheri, G. | 2019 | International Journal on Interactive Design and Manufacturing. | State of the art methodologies | methodology | optimization (lattice), mechanics, weight | ||||
| 2019 | 296 | Design Considerations of Heat Guides Fabricated Using Additive Manufacturing for Enhanced Heat Transfer in Electrical Machines | Wrobel, R., Hussein, A. | 2018 | 2018 IEEE Energy Conversion Congress and Exposition, ECCE 2018, art. no. 8557559, pp. 6506-6513. | State of the art methodologies | methodology | termofluids | ||||
| 2019 | 297 | Towards design for precision additive manufacturing: A simplified approach for detecting heat accumulation | Ranjan, R., Ayas, C., Langelaar, M., Van Keulen, F. | 2018 | Proceedings - 2018 ASPE and euspen Summer Topical Meeting: Advancing Precision in Additive Manufacturing, pp. 29-34. | State of the art methodologies | methodology | Restrictions, optimization, tolerances | ||||
| 2019 | 298 | Design of a multi-sensor in-situ inspection system for additive manufacturing | Dickins, A., Widjanarko, T., Lawes, S., Stravroulakis, P., Leach, R. | 2018 | Proceedings - 2018 ASPE and euspen Summer Topical Meeting: Advancing Precision in Additive Manufacturing, pp. 248-252. | State of the art methodologies | others | electronica | ||||
| 2019 | 299 | The applicability of the 40 TRIZ principles in design for additive manufacturing | Kretzschmar, N., Chekurov, S. | 2018 | Annals of DAAAM and Proceedings of the International DAAAM Symposium, 29(1), pp. 888-893. | State of the art methodologies | methodology | Modified DFX for AM | ||||
| 2019 | 300 | A Novel Approach to Optimize the Design of Parts for Additive Manufacturing | Silva, F.J.G., Campilho, R.D.S.G., Gouveia, R.M., Pinto, G., Baptista, A. | 2018 | Procedia Manufacturing, 17, pp. 53-61. | State of the art methodologies | methodology | Optimization | ||||
| 2019 | 301 | Design of metallic bone by additive manufacturing | Alabort, E., Barba, D., Reed, R.C. | 2019 | Scripta Materialia, 164, pp. 110-114. | State of the art methodologies | methodology | Mechanics, medicine, optimization (topological, lattice) | ||||
| 2019 | 302 | Design for Six Sigma (DFSS) for additive manufacturing applied to an innovative multifunctional fan | Liverani, A., Caligiana, G., Frizziero, L., (...), Donnici, G., Dhaimini, K. | 2019 | International Journal on Interactive Design and Manufacturing. | State of the art methodologies | methodology | Modified DFX for AM | ||||
| 2019 | 303 | Understanding the role of additive manufacturing knowledge in stimulating design innovation for novice designers | Yang, S., Page, T., Zhao, Y.F. | 2018 | Proceedings of the ASME Design Engineering Technical Conference 4. | State of the art methodologies | methodology | DWAM, educational | ||||
| 2019 | 304 | Overhang constraint for topology optimization of self-supported compliant mechanisms considering additive manufacturing | Garaigordobil, A., Ansola, R., Veguería, E., Fernandez, I. | 2019 | CAD Computer Aided Design 109, pp. 33-48 | State of the art methodologies | methodology | Topology optimization, constraints | ||||
| 2019 | 305 | Process planning for combined additive and subtractive manufacturing technologies in a remanufacturing context | Le, V.T., Paris, H., Mandil, G. | 2017 | Journal of Manufacturing Systems 44, pp. 243-254 | State of the art methodologies | methodology | planning process, manufacturing multiprocess | ||||
| 2019 | 306 | Deposition path planning-integrated structural topology optimization for 3D additive manufacturing subject to self-support constraint | Liu, J., To, A.C. | 2017 | CAD Computer Aided Design 91, pp. 27-45 | State of the art methodologies | methodology | Topology optimization, constraints | ||||
| 2019 | 307 ( 195) | Design of graded lattice structure with optimized mesostructures for additive manufacturing | Wang, Y., Zhang, L., Daynes, S., (...), Feih, S., Wang, M.Y. | 2018 | Materials and Design 142, pp. 114-123 | Optimization (multiscale: lattice, topological) | ||||||
| 2019 | 308 ( 177) | Computational design and additive manufacturing of periodic conformal metasurfaces by synthesizing topology optimization with conformal mapping | Vogiatzis, P., Ma, M., Chen, S., Gu, X.D. | 2018 | Computer Methods in Applied Mechanics and Engineering 328, pp. 477-497 | Optimization (multiscale: lattice, topological) | ||||||
| 2019 | 309 | Coupling lattice structure topology optimization with design-dependent feature evolution for additive manufactured heat conduction design | Cheng, L., Liu, J., Liang, X., To, A.C. | 2018 | Computer Methods in Applied Mechanics and Engineering 332, pp. 408-439 | State of the art methodologies | methodology | Optimization (multiscale: lattice, topological), thermofluids | ||||
| 2019 | 310 ( 107) | Topology optimization considering overhang constraint in additive manufacturing | Zhang, K., Cheng, G., Xu, L. | 2019 | Computers and Structures 212, pp. 86-100 | Topology optimization, constraints | ||||||
| 2019 | 311 | Integrated Product, Production and Material Definition for Conventional versus Generative Manufacturing Technologies | Kaspar, J., Stoffels, P., Schneberger, J.-H., Vielhaber, M. | 2018 | Procedia CIRP 70, pp. 180-185 | State of the art methodologies | methodology | material selection and process, assessment, process planning, multi-process manufacturing, conceptual design | ||||
| 2019 | 312 | Modeling Key Characteristics in the Value Chain of Additive Manufacturing | Al-Meslemi, Y., Anwer, N., Mathieu, L. | 2018 | Procedia CIRP 70, pp. 90-95 | State of the art methodologies | methodology | Selected materials and process, conceptual design | ||||
| 2019 | 313 | Strategies for functionally graded lattice structures derived using topology optimisation for Additive Manufacturing | Panesar, A., Abdi, M., Hickman, D., Ashcroft, I. | 2018 | Additive Manufacturing 19, pp. 81-94 | State of the art methodologies | methodology | Topology optimization, mechanics | ||||
| 2019 | 314 | Fused Deposition Modelling based Printing of Full Complement Bearings | Harikrishnan, U., Soundarapandian, S. | 2018 | Procedia Manufacturing 26, pp. 818-825 | State of the art methodologies | methodology | ensemble, tolerance | ||||
| 2019 | 315 | Adaptive metamaterials by functionally graded 4D printing | Bodaghi, M., Damanpack, A.R., Liao, W.H. | 2017 | Materials and Design 135, pp. 26-36 | Optimization (functional, multiscale) | ||||||
| 2019 | 316 ( 280) | Creativity and productivity in product design for additive manufacturing: Mechanisms and platform outcomes of remixing | Friesike, S., Flath, C.M., Wirth, M., Thiesse, F. | 2018 | Journal of Operations Management | innovation/creativity, assembly/fusion | ||||||
| 2019 | 317 | Part decomposition and 2D batch placement in single-machine additive manufacturing systems | Oh, Y., Zhou, C., Behdad, S. | 2018 | Journal of Manufacturing Systems 48, pp. 131-139 | State of the art methodologies | methodology | optimization process, assembly | ||||
| 2019 | 318 | Production scheduling and nesting in additive manufacturing | Chergui, A., Hadj-Hamou, K., Vignat, F. | 2018 | Computers and Industrial Engineering 126, pp. 292-301 | State of the art methodologies | methodology | Optimization of process (heuristic) | ||||
| 2019 | 319 | Effects of hollow structures in sand mold manufactured using 3D printing technology | Deng, C., Kang, J., Shangguan, H., (...), Huang, T., Liu, Z. | 2018 | Journal of Materials Processing Technology 255, pp. 516-523 | State of the art methodologies | methodology | design rules, experimental, molds | ||||
| 2019 | 320 | Consolidating spare parts for asset maintenance with additive manufacturing | Knofius, N., van der Heijden, M.C., Zijm, W.H.M. | 2019 | International Journal of Production Economics 208, pp. 269-280 | State of the art methodologies | methodology | maintenance, cost analysis | ||||
| 2019 | 321 | A fully developed flow thermofluid model for topology optimization of 3D-printed air-cooled heat exchangers | Haertel, J.H.K., Nellis, G.F. | 2017 | Applied Thermal Engineering 119, pp. 10-24 | State of the art methodologies | methodology | Optimization (topological), thermofluids, detail | ||||
| 2019 | 322 | Experimental characterization of an additively manufactured heat exchanger for dry cooling of power plants | Arie, M.A., Shooshtari, A.H., Ohadi, M.M. | 2018 | Applied Thermal Engineering 129, pp. 187-198 | State of the art methodologies | methodology | experimental, termofluidos, detail | ||||
| 2019 | 323 | Development of an additive manufacturing-enabled compact manifold microchannel heat exchanger | Tiwari, R., Andhare, R.S., Shooshtari, A., Ohadi, M. | 2019 | Applied Thermal Engineering pp. 781-788 | State of the art methodologies | methodology | termofluids | ||||
| 2019 | 324 | Additive manufacturing (3D printing): A review of materials, methods, applications and challenges | Ngo, T.D., Kashani, A., Imbalzano, G., Nguyen, K.T.Q., Hui, D. | 2018 | Composites Part B: Engineering 143, pp. 172-196 | State of the art methodologies | methodology | state of the art. | ||||
| 2019 | 325 | Additive manufacturing: scientific and technological challenges, market uptake and opportunities | Tofail, S.A.M., Koumoulos, E.P., Bandyopadhyay, A., (...), O'Donoghue, L., Charitidis, C. | 2018 | Materials Today 21(1), pp. 22-37 | State of the art methodologies | methodology | state of the art. | ||||
| 2019 | 326 | Controllable and reversible tuning of material rigidity for robot applications | Wang, L., Yang, Y., Chen, Y., (...), Askounis, E., Pei, Q. | 2018 | Materials Today 21(5), pp. 563-576 | State of the art methodologies | methodology | State of the art, variable stiffness materials (robotics) | ||||
| 2019 | 327 ( F45) | Criteria selection for a comparative study of functional performance of Fused Deposition Modelling and Vacuum Casting processes | Valerga Puerta, A.P., Sanchez, D.M., Batista, M., Salguero, J. | 2018 | Journal of Manufacturing Processes 35, pp. 721-727 | State of the art methodologies | methodology, manufacturing | Process selection (comparison and analysis) | ||||
| 2019 | 328 | Cost- and energy-efficient manufacture of gears by laser beam melting | Kamps, T., Lutter-Guenther, M., Seidel, C., Gutowski, T., Reinhart, G. | 2018 | CIRP Journal of Manufacturing Science and Technology 21, pp. 47-60 | State of the art methodologies | methodology | economic and energy analysis, life cycle, hybrid processes, comparative | ||||
| 2019 | 329 | Laser additive manufacturing and bionics: Redefining lightweight design | Emmelmann, C., Sander, P., Kranz, J., Wycisk, E. | 2011 | Physics Procedia 12(PART 1), pp. 364-368 | State of the art methodologies | methodology | optimization (topological), weight, mechanics | ||||
| 2019 | 330 | A Direct Material Reuse Approach Based on Additive and Subtractive Manufacturing Technologies for Manufacture of Parts from Existing Components | Le, V.T., Paris, H., Mandil, G., Brissaud, D. | 2017 | Procedia CIRP 61, pp. 229-234 | State of the art methodologies | methodology | Reducing waste, process planning/management, hybrid process. | ||||
| 2019 | 331 | A design for additive manufacturing ontology to support manufacturability analysis | Kim, S., Witherell, P., Rosen, D.W., Ko, H. | 2018 | Proceedings of the ASME Design Engineering Technical Conference, 2A-2018. | State of the art methodologies | methodology | DFAM, DFX mod AM | ||||
| 2019 | 332 | Integrated Cross-Component Lightweight and Material-Oriented Development Methodology - The Embodiment Design Cycle | Kaspar, J., Vielhaber, M. | 2018 | Procedia CIRP 70, pp. 481-486 | State of the art methodologies | methodology | Basic design, weight, mechanics | ||||
| 2019 | 333 | Additive manufacturing of silicon based PneuNets as soft robotic actuators | Manns, M., Morales, J., Frohn, P. | 2018 | Procedia CIRP 72, pp. 328-333 | State of the art methodologies | methodology | DFAM, limitations and advantages, robotics | ||||
| 2019 | 334 ( de 237) | Integrated design-oriented framework for Resource Selection in Additive Manufacturing | Uz Zaman, U.K., Rivette, M., Siadat, A., Baqai, A.A. | 2018 | Procedia CIRP 70, pp. 96-101 | DFAM, material selection and processes | ||||||
| 2019 | 335 | Selection method for multiple performances evaluation during early design stages | Audoux, K., Segonds, F., Kerbrat, O., Aoussat, A. | 2018 | Procedia CIRP 70, pp. 204-210 | State of the art methodologies | methodology | Method of selection and evaluation (conceptual and basic design) assessment (manufacturability, innovation sustainability) | ||||
| 2019 | 336 | The development of a strategy for direct part reuse using additive and subtractive manufacturing technologies | Le, V.T., Paris, H., Mandil, G. | 2018 | Additive Manufacturing 22, pp. 687-699 | State of the art methodologies | methodology | planning process, manufacturing multiprocess | ||||
| 2019 | 337 ( 266) | Digital design and nonlinear simulation for additive manufacturing | Weeger, O., Boddeti, N., Yeung, S.-K., Kaijima, S., Dunn, M.L. | 2019 | Additive Manufacturing 25, pp. 39-49 | optimization (lattice), mechanics | ||||||
| 2019 | 338 | Additive manufacturing — A review of 4D printing and future applications | Mitchell, A., Lafont, U., Hołyńska, M., Semprimoschnig, C. | 2018 | Additive Manufacturing 24, pp. 606-626 | State of the art methodologies | methodology | State of the art, optimization (lattice, topology), mechanics | ||||
| 2019 | 339 | Invited review article: Where and how 3D printing is used in teaching and education | Ford, S., Minshall, T. | 2019 | Additive Manufacturing 25, pp. 131-150 | State of the art methodologies | methodology | State of the art, DWAM, educational | ||||
| 2019 | 340 | Designing for Big Area Additive Manufacturing | Roschli, A., Gaul, K.T., Boulger, A.M., (...), Blue, F., Borish, M. | 2019 | Additive Manufacturing 25, pp. 275-285 | State of the art methodologies | methodology | design rules | ||||
| 2019 | 341 ( 124) | An additive manufacturing oriented design approach to mechanical assemblies | Sossou, G., Demoly, F., Montavon, G., Gomes, S. | 2018 | Journal of Computational Design and Engineering 5(1), pp. 3-18 | assembly, mechanic | ||||||
| 2019 | 342 ( 127) | Design for manufacturing to design for Additive Manufacturing: Analysis of implications for design optimality and product sustainability | Gebisa, A.W., Lemu, H.G. | 2017 | Procedia Manufacturing 13, pp. 724-731 | optimization, sustainability | ||||||
| 2019 | 343 | Topology optimization aided structural design: Interpretation, computational aspects and 3D printing | Kazakis, G., Kanellopoulos, I., Sotiropoulos, S., Lagaros, N.D. | 2017 | Heliyon 3(10),e00431 | State of the art methodologies | methodology | topological optimization, mechanics, weight | ||||
| 2019 | 344 | Additive Manufacturing - Considerations on Geometric Accuracy and Factors of Influence | Umaras, E., Tsuzuki, M.S.G. | 2017 | IFAC PapersOnLine 50-1 (2017) 14940–14945 | State of the art methodologies | methodology | Tolerances and roughness | ||||
| 2019 | 345 | Knowledge-based design of artificial neural network topology for additive manufacturing process modeling: A new approach and case study for fused deposition modeling | Nagarajan, H.P.N., Mokhtarian, H., Jafarian, H., (...), Gary Wang, G., Haapala, K.R. | 2019 | Journal of Mechanical Design, Transactions of the ASME, 141(2), art. no. 021705. | State of the art methodologies | methodology | optimization (neural networks, heuristic, topological), database | ||||
| 2019 | 346 | Rapid Manufacturing SLS® Design Guide | 3D SYSTEMS | 2016 | - | State of the art methodologies | methodology | design rules | ||||
| 2019 | 347 | Design for additive manufacturing: A creative approach | Rias, A.L., Bouchard, C., Segonds, F., Abed, S. | 2016 | Proceedings of International Design Conference, DESIGN DS 84, pp. 411-420 | State of the art methodologies | methodology | Creative, DFAM, creative (innovation), state of the art (it is research but has enough reference) | ||||
| 2019 | 348 | Generative design method for lattice structure with hollow struts of variable wall thickness | Wang, Y., Jing, S., Liu, Y., (...), Qie, L., Xing, H. | 2018 | Advances in Mechanical Engineering 10(3) | State of the art methodologies | methodology | Optimization (lattice), mechanics | ||||
| 2019 | 349 | A review of synthesis methods for additive manufacturing | Rosen, D.W. | 2016 | Virtual and Physical Prototyping 11(4), pp. 305-317 | State of the art methodologies | methodology | State of the art, Optimization (lattice, topological, shape) | ||||
| 2019 | 350 | A review on composite materials and process parameters optimisation for the fused deposition modelling process | Mohan, N., Senthil, P., Vinodh, S., Jayanth, N. | 2017 | Virtual and Physical Prototyping 12(1), pp. 47-59 | State of the art methodologies | methodology, manufacturing | Optimization (parameters, composite materials) | ||||
| 2019 | 351 | Additive manufacturing-integrated hybrid manufacturing and subtractive processes: Economic model and analysis | Manogharan, G., Wysk, R.A., Harrysson, O.L.A. | 2016 | International Journal of Computer Integrated Manufacturing 29(5), pp. 473-488 | State of the art methodologies | methodology | economic analysis, hybrid processes | ||||
| 2019 | 352 | Additive manufacturing management: a review and future research agenda | Khorram Niaki, M., Nonino, F. | 2017 | International Journal of Production Research 55(5), pp. 1419-1439 | State of the art methodologies | methodology, environment | State of the art, management, life cycle, economy, future business opportunities. | ||||
| 2019 | 353 ( 158) | Design optimization and validation of high-performance heat exchangers using approximation assisted optimization and additive manufacturing | Bacellar, D., Aute, V., Huang, Z., Radermacher, R. | 2017 | Science and Technology for the Built Environment 23(6), pp. 896-911 | fluid terms, optimization | ||||||
| 2019 | 354 | Design for manufacturing and assembly/disassembly: joint design of products and production systems | Battaïa, O., Dolgui, A., Heragu, S.S., Meerkov, S.M., Tiwari, M.K. | 2018 | International Journal of Production Research 56(24), pp. 7181-7189 | State of the art methodologies | methodology | State of the art (research article with many references), DFAM, assembly, modified DFX for AM. | ||||
| 2019 | 355 ( 138) | FDM for composite tooling Desin Guide | STRATASYS | - | - | DFAM | ||||||
| 2019 | 356 | FDM for composite tooling 2.0 Desin Guide | STRATASYS | - | - | State of the art methodologies | methodology | DFAM | ||||
| 2019 | 357 | Designing for additive manufacturing technologies: a design research methodology | Silvina Félix, Nuno Dias & Violeta Clemente | 2017 | The Design Journal 20:sup1, S4754- S4757 | State of the art methodologies | methodology | DFAM | ||||
| 2019 | 358 | Design of Three-Dimensional, Triply Periodic Unit Cell Scaffold Structures for Additive Manufacturing | Mohammed, M.I., Gibson, I. | 2018 | Journal of Mechanical Design, Transactions of the ASME 140(7),071701 | State of the art methodologies | methodology | Optimization (lattice) | ||||
| 2019 | 359 | Design for Additively Manufactured Lightweight Structure: A Perspective | L. Yang1, O. L. A. Harrysson2, D. Cormier3, H. West2, S. Zhang1, H. Gong4, B. Stucker5 | 2016 | Solid Freeform Fabrication 2016: Proceedings of the 26th Annual International Solid Freeform Fabrication Symposium – An Additive Manufacturing Conference | State of the art methodologies | methodology | Optimization (lattice, topological), mechanics, weight | ||||
| 2019 | 360 | Simulation based method considering design for additive manufacturing and supply chain An empirical study of lamp industry | Chiu, M.-C., Lin, Y.-H. | 2016 | Industrial Management and Data Systems 116(2), pp. 322-348 | State of the art methodologies | methodology | economy, administration, management | ||||
| 2019 | 361 | Cooling system for 0.1 kN thrust micro-engines: Concept design using additive manufacturing | Ugolotti, M., Sharma, M., Williams, Z., (...), Ouwerkerk, J., Turner, M. | 2017 | 58th AIAA/ASCE/AHS/ASC Structures, Structural Dynamics, and Materials Conference, 2017 | State of the art methodologies | methodology | termofluids | ||||
| 2019 | 362 | Multiple material additive manufacturing - Part 1: A review | Vaezi, M., Chianrabutra, S., Mellor, B., Yang, S. | 2013 | Virtual and Physical Prototyping 8(1), pp. 19-50 | State of the art methodologies | methodology | multimaterial | ||||
| 2019 | 363 | Rapid Prototyping-Distance Delivery Tools | Ismail Fidan, Birhan Isik | 2009 | US – TURKEY Workshop On Rapid Technologies, September 24 – 24, 2009 | DWAM, educational, online | ||||||
| 2019 | 364 | Review of Heat Exchangers Enabled by Polymer and Polymer Composite Additive Manufacturing | Deisenroth, D.C., Moradi, R., Shooshtari, A.H., (...), Bar-Cohen, A., Ohadi, M. | 2018 | Heat Transfer Engineering 39(19), pp. 1652-1668 | State of the art methodologies | methodology | State of the art, thermofluids | ||||
| 2019 | 365 | Additive manufacturing: Current state, future potential, gaps and needs, and recommendations | Huang, Y., Leu, M.C., Mazumder, J., Donmez, A. | 2015 | Journal of Manufacturing Science and Engineering, Transactions of the ASME, 137(1),014001 | State of the art methodologies | methodology | State of the art AM | ||||
| 2019 | 366 | CAD and AM-fabricated moulds for fast cranio-maxillofacial implants manufacture | Ruiz-Huerta, L., Almanza-Arjona, Y.C., Caballero-Ruiz, A., (...), Díaz-Aguirre, C.M., Echevarría Y Pérez, E. | 2016 | Rapid Prototyping Journal 22(1), pp. 31-39 | State of the art methodologies | methodology | medical | ||||
| 2019 | 367 | A statistical method for build orientation determination in additive manufacturing | Zhang, Y., Harik, R., Fadel, G., Bernard, A. | 2019 | Rapid Prototyping Journal 25(1), pp. 187-207 | State of the art methodologies | methodology | Tolerances and roughness | ||||
| 2019 | 368 | The FaaS system using additive manufacturing for personalized production | Kang, H.S., Noh, S.D., Son, J.Y., (...), Park, J.H., Lee, J.Y. | 2018 | Rapid Prototyping Journal 24(9), pp. 1486-1499 | State of the art methodologies | methodology, manufacturing | online manufacturing | ||||
| 2019 | 369 | Smart materials in additive manufacturing: state of the art and trends | Gardan, J. | 2019 | Virtual and Physical Prototyping 14(1), pp. 1-18 | State of the art methodologies | methodology | State of the art, optimization (lattice) | ||||
| 2019 | 370 | Standardised product development for technology integration of additive manufacturing | Rohde, J., Jahnke, U., Lindemann, C., Kruse, A., Koch, R. | 2019 | Virtual and Physical Prototyping 14(2), pp. 141-147 | State of the art methodologies | methodology | Design and process selection, production chain. | ||||
| 2021 | 371 | 3D printing: Printing precision and application in food sector | Zhenbin Liu and Min Zhang and Bhesh Bhandari and Yuchuan Wang | 2017 | Journal Article published Nov 2017 in Trends in Food Science & Technology volume 69 on pages 83 to 94 | State of the art methodologies | methodology | applications in the food industry | ||||
| 2021 | 372 | Additive Manufacturing Principles and Capabilities Cards | K Blake Perez and Kristin Lee Wood | 2019 | State of the art methodologies | methodology | design rule, innovation | |||||
| 2021 | 373 | Additive Manufacturing (AM) Design Principle Cards | Perez, K Blake and Wood, Kristin | 2019 | State of the art methodologies | methodology | design rule, innovation | |||||
| 2021 | 374 | Knowledge-Based Design of Artificial Neural Network Topology for Additive Manufacturing Process Modeling: A New Approach and Case Study for Fused Deposition Modeling | Hari P. N. Nagarajan and Hossein Mokhtarian and Hesam Jafarian and Saoussen Dimassi and Shahriar Bakrani-Balani and Azarakhsh Hamedi and Eric Coatan{\'{e}}a and G. Gary Wang and Karl R. Haapala | 2019 | Journal Article published 1 Feb 2019 in Journal of Mechanical Design volume 141 issue 2 | State of the art methodologies | methodology | Knowledge Database for FDM, Neural Network | ||||
| 2021 | 375 | Product Design for Manufacture and Assembly, Third Edition | Geoffrey Boothroyd, Peter Dewhurst, Winston A. Knight | 2011 | CRC Press, Taylor & Francis Group | State of the art methodologies | methodology | DFMA, state of the art review, design rule | ||||
| 2021 | 376 | Engineering design | Dieter, George Ellwood and Schmidt, Linda C and others | 2009 | McGraw-Hill Higher Education Boston | State of the art methodologies | methodology | Conventional design theory, state of the art review, design rule. | ||||
| 2021 | 377 | Engineering design: a systematic approach | Pahl, Gerhard and Beitz, Wolfgang | 2013 | Springer Science \& Business Media | State of the art methodologies | methodology | Conventional design theory, state of the art review, design rule. | ||||
| 2021 | 378 | The mechanical design process | Ullman, David G | 2010 | McGraw-Hill New York | State of the art methodologies | methodology | Conventional design theory, state of the art review, design rule. | ||||
| 2021 | 379 | Structural analysis of wing ribs obtained by additive manufacturing | Pedro Miguel Cardoso Carneiro and Pedro Gamboa | 2019 | Journal Article published 13 May 2019 in Rapid Prototyping Journal volume 25 issue 4 on pages 708 to 720 | State of the art methodologies, mechanical modeling, failure theory. | mechanics, failure theory | simulation, design of reinforcements for wings, aerospace | ||||
| 2021 | 380 | Classification of challenges in 3D printing for combined electrochemical and microfluidic applications: a review | Arivarasi A. and Anand Kumar | 2019 | Journal Article published 12 Aug 2019 in Rapid Prototyping Journal volume 25 issue 7 on pages 1328 to 1346 | State of the art methodologies | methodology | State of the art, electrochemistry, microfluidics | ||||
| 2021 | 381 | Investigation of professional design practice: a framework for designing plastic consumer products for additive manufacturing | Wei Liu, Zicheng Zhu, Songhe Ye, Xiaoneng Jin, Guanghe Yan | 2019 | Int. J. Materials and Product Technology, Vol. 58, Nos. 2/3, 2019 | State of the art methodologies | methodology | Industrial practices and professions in AM, design rules. | ||||
| 2021 | 382 | Fused deposition modelling: a review | Swapnil Vyavahare and Soham Teraiya and Deepak Panghal and Shailendra Kumar | 2019-2020 | Journal Article published 6 Jan 2020 in Rapid Prototyping Journal volume 26 issue 1 on pages 176 to 201 | State of the art methodologies, mechanical modeling, optimization, surface modeling, Manufacturing process cases (general and specific) | Methodology, mechanics, surface, manufacturing, dimension | Mechanical characterization, design rule, finish, process chain, multiprocess, tolerances. | ||||
| 2021 | 383 | 3D printing: a critical review of current development and future prospects | Md. Hazrat Ali and Shaheidula Batai and Dastan Sarbassov | 2019 | Journal Article published 8 Jul 2019 in Rapid Prototyping Journal volume 25 issue 6 on pages 1108 to 1126 | State of the art methodologies, mechanical modeling, optimization, surface modeling, Manufacturing process cases (general and specific) | Methodology, mechanics, surface, manufacturing, dimension, optimization | Mechanical characterization, design rule, finishing, tolerances, optimization. | ||||
| 2021 | 384 | Methods and materials for additive manufacturing: A critical review on advancements and challenges | M Bhuvanesh Kumar and P Sathiya | 2021 | Thin-Walled Structures Volume 159, February 2021, 107228 | State of the art methodologies, mechanical modeling, optimization, surface modeling, manufacturing process cases (general and specific), medical applications, dimensional modeling. | Methodology, mechanics, surface, manufacturing, dimension, optimization, medicine. | Mechanical characterization, design rule, finishing, tolerances, optimization, biomaterials, tissue engineering, tissue anchoring. | ||||
| 2021 | 385 | A review on quality control in additive manufacturing | Hoejin Kim and Yirong Lin and Tzu-Liang Bill Tseng | 2018 | Journal Article published 9 Apr 2018 in Rapid Prototyping Journal volume 24 issue 3 on pages 645 to 669 | State of the art methodologies, surface modeling, Cases of manufacturing processes (general and specific), dimensional modeling. | Methodology, surface, manufacturing, dimension | Design rule, finish, tolerances | ||||
| 2021 | 386 | Current status and future directions of fused filament fabrication | Sunpreet Singh and Gurminder Singh and Chander Prakash and Seeram Ramakrishna | 2020 | Journal of Manufacturing Processes Volume 55, July 2020, Pages 288-306 | State of the art methodologies, mechanical modeling, optimization, surface modeling, Manufacturing process cases (general and specific) | Methodology, mechanics, surface, manufacturing, dimension | Mechanical characterization, design rule, finish, process chain, multiprocess, tolerances. | ||||
| 2021 | 387 | CAD-based design and pre-processing tools for additive manufacturing | Botao Zhang and Archak Goel and Omkar Ghalsasi and Sam Anand | 2019 | Journal of Manufacturing Systems Volume 52, Part B, July 2019, Pages 227-241 | State of the art methodologies, surface modeling, Cases of manufacturing processes (general and specific) | Methodology, surface, manufacturing | Design rule, finish, manufacturability | ||||
| 2021 | 388 | Methodology for design process of a snap-fit joint made by additive manufacturing | Emilio A. Ramírez, Fausto Caicedo, Jorge Hurel, Carlos G. Helguero, Jorge Luis Amaya | 2019 | Journal Article published 2019 in Procedia CIRP volume 79 on pages 113 to 118 | State of the art methodologies | methodology, assembly | rule of design, assembly | ||||
| 2021 | 389 | Detailed design process and assembly considerations for snap-fit joints using additive manufacturing | Jorge Luis Amaya, Emilio A. Ramírez, Galarza F. Maldonado, Jorge Hurel | 2019 | Journal Article published 2019 in Procedia CIRP volume 84 on pages 680 to 687 | State of the art methodologies | methodology, assembly | rule of design, assembly | ||||
| 2021 | 390 | Multi-objective optimization approach in design for additive manufacturing for fused deposition modeling | Elnaz Asadollahi-Yazdi, Julien Gardan, Pascal Lafon | 2019 | Journal Article published 10 Jun 2019 in Rapid Prototyping Journal volume 25 issue 5 on pages 875 to 887 | State of the art methodologies, optimization, surface modeling, mechanical modeling. | Methodology, surface, mechanics, optimization, manufacturing | Mechanical characterization, finish, manufacturability/manufacturing. | ||||
| 2021 | 391 | Design for additive manufacturing – a review of available design methods and software | Anton Wiberg, Johan Persson, Johan Ölvander | 2019 | Journal Article published 8 Jul 2019 in Rapid Prototyping Journal volume 25 issue 6 on pages 1080 to 1094 | State of the art methodologies | methodology | review of methodologies and computer tools, software | ||||
| 2021 | 392 | A design for additive manufacturing case study: fingerprint stool on a BigRep ONE | James I. Novak, Jonathon O’Neill | 2019 | Journal Article published 8 Jul 2019 in Rapid Prototyping Journal volume 25 issue 6 on pages 1069 to 1079 | State of the art methodologies | methodology | Design rule, finish, manufacturability, costs | ||||
| 2021 | 393 | Personalized design of part orientation in additive manufacturing | Cong Yu, LongFei Qie, ShiKai Jing, Yan Yan | 2019 | Journal Article published 11 Nov 2019 in Rapid Prototyping Journal volume 25 issue 10 on pages 1647 to 1660 | State of the art methodologies, optimization, surface modeling, dimensional modeling, market study and environment. | Methodology, surface, tolerances, dimension, optimization, manufacturing, cost. | Methodology, surface, tolerances, dimension, optimization, manufacturing, cost. | ||||
| 2021 | 394 | Design Innovation With Additive Manufacturing: A Methodology | K. Blake Perez, Carlye A. Lauff, Bradley A. Camburn, Kristin L. Wood | 2019 | Proceedings Article published 18 Aug 2019 in Volume 7: 31st International Conference on Design Theory and Methodology | State of the art methodologies | methodology | Methodology, innovation, design rules | ||||
| 2021 | 395 | Design Principle Cards: Toolset to Support Innovations With Additive Manufacturing | Carlye A. Lauff and K. Blake Perez and Bradley A. Camburn and Kristin L. Wood | 2019 | Proceedings Article published 18 Aug 2019 in Volume 4: 24th Design for Manufacturing and the Life Cycle Conference; 13th International Conference on Micro- and Nanosystems | State of the art methodologies | methodology | design rule, innovation | ||||
| 2021 | 396 | Choice between virtual model and prototype in additive manufacturing design process | Thanh Hoang Vo, Guy Prudhomme, Philippe Marin, Frédéric Vignat | 2019 | DYNA-BILBAO | State of the art methodologies, optimization. | Methodology, optimization, costs, manufacturability | Methodology for prototype selection for testing, costs, manufacturability. | ||||
| 2021 | 397 | A new methodology for design and manufacturing of a customized silicone partial foot prosthesis using indirect additive manufacturing | Osama Abdelaal and Saied Darwish and Khaled Abd Elmougoud and Saleh Aldahash | 2019 | The International Journal of Artificial Organs 2019, Vol. 42(11) 645–657 | State of the art methodologies, Cases of production and manufacturing processes (general and specific), Medical applications. | methodology, medicine | prosthesis, fabricability to English is prosthesis, fabricability. | ||||
| 2021 | 398 | Design and Manufacturing Strategies for Fused Deposition Modelling in Additive Manufacturing: A Review | Hugo I. Medellin-Castillo and Jorge Zaragoza-Siqueiros | 2019 | Journal Article published Dec 2019 in Chinese Journal of Mechanical Engineering volume 32 issue 1 | State of the art methodologies, mechanical modeling, optimization, surface modeling, Manufacturing process cases (general and specific) | Methodology, mechanics, surface, manufacturing, dimension | Mechanical characterization, design rule, finish, process chain, multiprocess, tolerances. | ||||
| 2021 | 399 | Evaluating the Potential of Design for Additive Manufacturing Heuristic Cards to Stimulate Novel Product Redesigns | Alexandra Blösch-Paidosh and Saeema Ahmed-Kristensen and Kristina Shea | 2019 | Proceedings Article published 18 Aug 2019 in Volume 2A: 45th Design Automation Conference | State of the art methodologies | methodology | design rule, innovation | ||||
| 2021 | 400 | An economic analysis comparing the cost feasibility of replacing injection molding processes with emerging additive manufacturing techniques | Matthew Franchetti and Connor Kress | 2017 | Int J Adv Manuf Technol (2017) 88:2573–2579 | State of the art methodologies | methodology, costs | methodology, costs | ||||
| 2021 | 401 | Evaluation of technical and economic feasibility of additive manufacturing technology: evidences from a case study | Zanardini, M and Bacchetti, A and Adrodegari, F | 2016 | Industrial Systems Engineering | State of the art methodologies | methodology, costs | methodology, costs SLS FDM | ||||
| 2021 | 402 | Environmental and Economic Implications of Distributed Additive Manufacturing: The Case of Injection Mold Tooling | Runze Huang and Matthew E. Riddle and Diane Graziano and Sujit Das and Sachin Nimbalkar and Joe Cresko and Eric Masanet | 2017 | Journal Article published Nov 2017 in Journal of Industrial Ecology volume 21 issue S1 on pages S130 to S143 | State of the art methodologies | Methodology, costs, environment | Methodology, costs, environment | ||||
| 2021 | 403 | Additive manufacturing: status and opportunities | Gupta, Nayanee and Weber, Christopher and Newsome, Sherrica | 2012 | Science and Technology Policy Institute, Washington | State of the art methodologies | methodology | state of the art. | ||||
| 2021 | 404 | Design for additive manufacturing: a comprehensive review of the tendencies and limitations of methodologies | Luis Lisandro Lopez Taborda, Heriberto Maury, Jovanny Pacheco | 2021 | Journal Article published 4 Jun 2021 in Rapid Prototyping Journal volume ahead-of-print issue ahead-of-print | State of the art methodologies | methodology | state of the art. | ||||
| 2023 | 405 | Beautiful and Functional: A Review of Biomimetic Design in Additive Manufacturing | du Plessis, A., Broeckhoven, C., Yadroitsava, I., (...), Kunju, R., Bhate, D. | 2019 | Additive Manufacturing, 27, pp. 408-427. | State of the art methodologies | methodology | state of the art. | ||||
| 2023 | 406 | A design framework for additive manufacturing | Bikas, H., Lianos, A.K., Stavropoulos, P. | 2019 | Additive Manufacturing, Volume 27, May 2019, Pages 408-427 | State of the art methodologies | methodology | state of the art. | ||||
| 2023 | 407 | Methodology for design process of a snap-fit joint made by additive manufacturing | Ramírez, E.A., Caicedo, F., Hurel, J., Helguero, C.G., Amaya, J.L. | 2019 | Procedia CIRP, 79, pp. 113-118 | State of the art methodologies | design methodology | Functional/assembly/joints | ||||
| 2023 | 408 | Industrial Case Studies of Design for Plastic Additive Manufacturing for End-Use Consumer Products | Liu, W., Zhu, Z., Ye, S. | 2019 | 3D Printing and Additive Manufacturing, 6(6), pp. 281-292. | State of the art methodologies | methodology | state of the art. | ||||
| 2018 | TF0 | Mechanics of Composite Materials, Second Edition | Autar Kaw | 2005 | CRC (PRESS) | failure theory | failure theory | composite materials | ||||
| 2018 | TF1 | Strength and failure mechanism in 3D printed parts | Bishwonath Adhikari | 2017 | Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Technology, AALTO UNIVERSITY SCHOOL OF ENGINEERING Department of Mechanics of Material | failure theory | failure theory | failure theory fdm | ||||
| 2018 | TF2 | Evaluating Mechanical Properties and Failure Mechanisms of Fused Deposition Modeling Acrylonitrile Butadiene Styrene Parts | M. S. Uddin et all | 2017 | Journal of Manufacturing Science and Engineering AUGUST 2017, Vol. 139 | failure theory | failure theory | Theory of failure FDM/fractography | ||||
| 2018 | TF3 | An assessment of the effect of printing orientation, density, and filler pattern on the compressive performance of 3D printed ABS structures by fuse deposition | G. Domínguez-Rodríguez1 & J. J. Ku-Herrera2 & A. Hernández-Pérez3 | 2017 | Int J Adv Manuf Technology | failure theory | failure theory | The translated value of teoria de falla fdm/ compresion in English is failure theory fdm/ compression. | ||||
| 2018 | TF4 | Mechanical properties and failure mechanisms of sandwich panels with ultra-lightweight three-dimensional hierarchical lattice cores | Qianqian Wu a , Ying Gao a , Xingyu Wei a , Davood Mousanezhad b , Li Ma a , Ashkan Vaziri b , Jian Xiong a , | 2018 | International Journal of Solids and Structures 132–133 (2018) 171–187 | failure theory | failure theory | mechanical properties/failure mechanism/compression | ||||
| 2018 | TF5 | Damage evolution and failure mechanisms in additively manufactured stainless steel | HollyD.Carlton a,n, AbdelHaboub c, GilbertF.Gallegos a, DilworthY.Parkinson b, Alastair A.MacDowell b | 2016 | Materials Science&EngineeringA651(2016)406–414 | failure theory | failure theory | Failure mechanism/fractography | ||||
| 2018 | TF6 | FAILURE CRITERION FOR SLS ADDITIVE MANUFACTURED PARTS | P. Obst, M. Launhardt and D. Drummer, LKT, Friedrich Alexander University, Erlangen, Germany P. V. Osswald, Technical University of Munich, Munich, Germany T. A. Osswald, Polymer Engineering Center, University of Wisconsin-Madison, WI, USA | 2017 | failure theory | failure theory | sls failure theory | |||||
| 2021 | TF6B | Failure criterion for PA12 SLS additive manufactured parts | P. Obst and M. Launhardt and D. Drummer and P.V. Osswald and T.A. Osswald | 2018 | Additive Manufacturing 21 (2018) 619–627 | failure theory | failure theory | sls failure theory | ||||
| 2018 | TF7 | Strength-based topology optimization for anisotropic parts | Amir M. Mirzendehdel, Behzad Rankouhi, Krishnan Suresh | 2018 | Additive Manufacturing 19 (2018) 104–113 | failure theory | failure theory | Mechanical resistance/topological optimization/failure theory FDM | ||||
| 2018 | TF8 | The effect of anisotropy on the optimization of additively manufactured lattice structures | Tino Stankovi´c∗, Jochen Mueller, Kristina Shea | 2017 | Additive Manufacturing 17 (2017) 67–76 | failure theory | failure theory | mechanical resistance/optimization | ||||
| 2018 | TF9 | Effect of build orientation on the mechanical reliability of 3D printed ABS | Özgür Keleş, Caleb Wayne Blevins, Keith J. Bowman | 2017 | Rapid Prototyping Journal, Vol. 23 Issue: 2, pp.320-328, | failure theory | failure theory | fracture mechanics | ||||
| 2018 | TF10 | Influence of meso-structure and chemical composition on FDM 3D-printed parts | Gianluca Alaimo, Stefania Marconi, Luca Costato, Ferdinando Auricchio* | 2017 | Composites Part B 113 (2017) 371e380 | failure theory | failure theory | Theory of failure FDM | ||||
| 2018 | TF11 | Tsai-Wu Analysis of a Thin-Walled 3D-Printed Polylactic Acid (PLA) Structural Bracket | Ruiqi Chen, Stanford University; Ashwin Ramachandran, Stanford University; Cheng Liu, Stanford University; Fu-Kuo Chang, Stanford University; Debbie Senesky, Stanford | 2017 | 58th AIAA/ASCE/AHS/ASC Structures, Structural Dynamics, and Materials Conference Grapevine, Texas | failure theory | failure theory | Theory of failure FDM | ||||
| 2018 | TF12 | Investigation of adhesion strength of metallization on thermoplastic and ceramic substrates | Sven Brinkhues, Akhil Kanthamneni, Andreas Brose | 2016 | 2016 12th international congress molded interconnect devices - scientific proceedings, MID 2016 7738935 | failure theory | failure theory | Adhesion resistance | ||||
| 2018 | TF13 | Fracture mechanical characterization and lifetime estimation of near-homogeneous components produced by fused filament fabrication | Florian Arbeitera,*, Martin Spoerkb, Johannes Wienera, Anja Goscha, Gerald Pintera | 2018 | Polymer Testing | failure theory | failure theory | fracture mechanics | ||||
| 2019 | TF14 | A Method to Improve the Fracture Toughness Using 3D Printing by Extrusion Deposition | Julien Gardan and Ali Makke and Naman Recho | 2016 | Procedia Structural Integrity | failure theory | - | fracture mechanics | ||||
| 2019 | TF15 | The impact of print orientation and raster pattern on fracture toughness in additively manufactured ABS | McLouth, T.D., Severino, J.V., Adams, P.M., Patel, D.N., Zaldivar, R.J. | 2017 | Additive Manufacturing 18, pp. 103-109 | failure theory | - | fracture mechanics | ||||
| 2019 | TF16 | Mechanical strength of welding zones produced by polymer extrusion additive manufacturing | Davis, C.S., Hillgartner, K.E., Han, S.H., Seppala, J.E. | 2017 | Additive Manufacturing 16, pp. 162-166 | failure theory | - | interlayer resistance | ||||
| 2019 | TF17 | Fracture resistance measurement of fused deposition modeling 3D printed polymers | Aliheidari, N., Tripuraneni, R., Ameli, A., Nadimpalli, S. | 2017 | Polymer Testing 60, pp. 94-101 | failure theory | - | fracture mechanics | ||||
| 2021 | TF18 | Fracture behavior of additively manufactured components: A review | Mohammad Reza Khosravani and Filippo Berto and Majid R. Ayatollahi and Tamara Reinicke | 2020 | Theoretical and Applied Fracture Mechanics Volume 109, October 2020, 102763 | failure theory | failure theory | fracture mechanics | ||||
| 2021 | TF19 | Fracture mechanics: fundamentals and applications | Anderson, Ted L | 2017 | CRC press | failure theory | failure theory | fracture mechanics | ||||
| 2021 | TF20 | THE STRESS ANALYSIS OF CRACKS HANDBOOKS | Tada, Hiroshi and Paris, P and Irwin, G | 2000 | ASME PRESS | failure theory | failure theory | fracture mechanics | ||||
| 2021 | TF21 | Fracture loads prediction of the modified 3D-printed ABS specimens under mixed-mode I/II loading | B. Ameri and F. Taheri-Behrooz and M.R.M. Aliha | 2020 | Engineering Fracture Mechanics 235 (2020) 107181 | failure theory | failure theory | fracture mechanics | ||||
| 2021 | TF22 | Failure surface development for ABS fused filament fabrication parts | Gerardo A. {Mazzei Capote} and Natalie M. Rudolph and Paul V. Osswald and Tim A. Osswald | 2019 | Additive Manufacturing 28 (2019) 169–175 | failure theory | failure theory | Static failure theory | ||||
| 2023 | TF22B | Validating a Failure Surface Developed for {ABS} Fused Filament Fabrication Parts through Complex Loading Experiments | Gerardo A. Mazzei Capote *, Alec Redmann and Tim A. Osswald | 2019 | J. Compos. Sci. 2019, 3, 49; doi:10.3390/jcs3020049 | failure theory | failure theory | Static failure theory | ||||
| 2021 | TF23 | A strength tensor based failure criterion with stress interactions | Paul V. Osswald and Tim A. Osswald | 2018 | Polymer Composites volume 39 issue 8 on pages 2826 to 2834 | failure theory | failure theory | Static failure theory | ||||
| 2021 | TF24 | A method to predict the ultimate tensile strength of 3D printing polylactic acid (PLA) materials with different printing orientations | Tianyun Yao and Zichen Deng and Kai Zhang and Shiman Li | 2019 | Composites Part B 163 (2019) 393–402 | failure theory | failure theory | static failure theory, sheet | ||||
| 2021 | TF25 | Fracture Resistance Analysis of 3D-Printed Polymers | Ali Zolfagharian and Mohammad Reza Khosravani and Akif Kaynak | 2020 | Polymers volume 12 issue 2 on page 302 | failure theory | failure theory | fracture mechanics | ||||
| 2021 | TF26 | Fracture and load-carrying capacity of 3D-printed cracked components | Mohammad Reza Khosravani and Ali Zolfagharian | 2020 | Extreme Mechanics Letters 37 (2020) 100692 | failure theory | failure theory | fracture mechanics | ||||
| 2021 | TF27 | Numerical and experimental studies of additively manufactured polymers for enhanced fracture properties | J. Li and S. Yang and D. Li and V. Chalivendra | 2018 | Engineering Fracture Mechanics 204 (2018) 557–569 | failure theory, mechanical modeling | failure theory, mechanics | Fracture mechanics, characterization, simulation | ||||
| 2021 | TF28 | Fracture of 3D-printed polymers: Crucial role of filament-scale geometric features | James Allum and Andrew Gleadall and Vadim V. Silberschmidt | 2020 | Engineering Fracture Mechanics 224 (2020) 106818 | failure theory | failure theory | fracture mechanics | ||||
| 2021 | TF29 | The Essential Work of Fracture parameters for 3D printed polymer sheets | I.I. Cuesta and E. Martinez-Pañeda and A. Díaz and J.M. Alegre | 2019 | Materials and Design 181 (2019) 107968 | failure theory | failure theory | fracture mechanics | ||||
| 2021 | TF30 | Interlayer adhesion and fracture resistance of polymers printed through melt extrusion additive manufacturing process | Nahal Aliheidari and Josef Christ and Rajasekhar Tripuraneni and Siva Nadimpalli and Amir Ameli | 2018 | Materials and Design 156 (2018) 351–361 | failure theory, mechanical modeling | failure theory, mechanics | Fracture mechanics, characterization, simulation | ||||
| 2021 | TF31 | Modeling the strength of 3D printed parts | Johnny Wikström | 2015 | Aalto University, School of Engineering, Mechanical Engineering | failure theory | failure theory | Static failure theory | ||||
| 2021 | TF32 | Numerical Prediction of 3D Printed Specimens Based on a Strengthening Method of Fracture Toughness | Marouene Zouaoui, Carl Labergere, Julien Gardan, Ali Makke, Naman Recho, Quentin Alexandre, Pascal Lafon | 2019 | Procedia CIRP volume 81 on pages 40 to 44 | failure theory, mechanical modeling | failure theory, mechanics | Fracture mechanics, characterization, simulation | ||||
| 2017 | M1 | Fused deposition modeling with polypropylene | O.S. Carneiro, A.F. Silva, R. Gomes | 2015 | Materials & Design 83 (2015) 768–776 | mechanical modeling | mechanics | Additive | ||||
| 2017 | M2 | Fiber Bragg grating based investigation of residual strains in ABS parts fabricated by fused deposition modeling process | Antreas Kantaros, Dimitris Karalekas | 2013 | Materials and Design 50 (2013) 44–50 | Creation of new materials | mechanics | Residual stresses | ||||
| 2017 | M3 | Impact absorption capacity of 3D-printed components fabricated by fused deposition modelling | A. Tsouknidas, M. Pantazopoulos, I. Katsoulis, D. Fasnakis, S. Maropoulos, N.Michailidis | 2016 | Materials and Design 102 (2016) 41–44 | mechanical modeling | mechanics | Impact | ||||
| 2017 | M4 | Additive manufacturing and mechanical characterization of graded porosity scaffolds designed based on triply periodic minimal surface architectures | Afshar, M., Anaraki, A.P., Montazerian, H., Kadkhodapour, J. | 2016 | journal of the mechanical behavior of biomedical materials 62 (2016) 481–494 | mechanical modeling | mechanics | Porosity | ||||
| 2017 | M5 | Experimental characterization and analytical modelling of the mechanical behaviour of fused deposition processed parts made of ABS-M30 | Dario Croccolo, Massimiliano De Agostinis, Giorgio Olmi | 2013 | Computational Materials Science 79 (2013) 506–518 | mechanical modeling | mechanics | Modeling and additive | ||||
| 2017 | M6 | Influence of Fill Gap on Flexural Strength of Parts Fabricated by Curved Layer Fused Deposition Modeling | Hua Wei Guan, Monica Mahesh Savalani, Ian Gibson, Olaf Diegel | 2015 | Procedia Technology 20 ( 2015 ) 243 – 248 | mechanical modeling | mechanics | Flexion | ||||
| 2017 | M7 | Isotropic and anisotropic elasticity and yielding of 3D printed material | Zou, R., Xia, Y., Liu, S., (...), Hu, Q., Shan, C. | 2016 | Composites Part B 99 (2016) 506e513 | mechanical modeling | mechanics | Modeling | ||||
| 2017 | M8 | Parametric appraisal of mechanical property of fused deposition modelling processed parts | Anoop Kumar Sood, R.K. Ohdar, S.S. Mahapatra | 2010 | Materials and Design 31 (2010) 287–295 | mechanical modeling | mechanics | deer | ||||
| 2017 | M9 | Influence of inter-layer cooling time on the quasi-static properties of ABS components produced via Fused Deposition Modelling | M. Faes, E. Ferraris, D. Moens | 2016 | Procedia CIRP 42 ( 2016 ) 748 – 753 | mechanical modeling | mechanics | Cooling time | ||||
| 2017 | M10 | 3-D printing of multifunctional carbon nanotube yarn reinforced components | John M. Gardnera, Godfrey Sautib, Jae-Woo Kimb, Roberto J. Canoa, Russell A. Wincheskia, Christopher J. Stelter a, Brian W. Grimsleya, Dennis C. Workinga, Emilie J. Siochia,∗ | 2016 | Additive Manufacturing 12 (2016) 38–44 | mechanical modeling | mechanics | nanotubes | ||||
| 2017 | M11 | Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds | F.S.Senatovn, K.V.Niaza,M.Yu.Zadorozhnyy,A.V.Maksimkin, S.D.Kaloshkin,Y.Z.Estrin | 2016 | j o urnal of the mechanical behavior of biomedical materials 57 (2016) 139–148 | mechanical modeling | mechanics | Porosity | ||||
| 2017 | M12 | Development of in-house composite wire based feed stock filaments of fused deposition modelling for wear-resistant materials and structures | Rupinder Singh, Sunpreet Singh, Fernando Fraternali | 2016 | Composites Part B 98 (2016) 244e249 | Creation of new materials | mechanics | additive | ||||
| 2017 | M13 | Thermo-mechanical properties of a highly filled polymeric composites for Fused Deposition Modeling | M. Nikzad, S.H. Masood ⇑, I. Sbarski | 2011 | Materials and Design 32 (2011) 3448–3456 | Creation of new materials | mechanics | additive | ||||
| 2017 | M14 | Mechanical and thermal properties of ABS/montmorillonite nanocomposites for fused deposition modeling 3D printing | Zixiang Weng a,c, JianleiWang a,c, T. Senthil a, LixinWu a,b,⁎ | 2016 | Materials and Design 102 (2016) 276–283 | Creation of new materials | mechanics | additive | ||||
| 2017 | M15 | Fused deposition modeling of novel scaffold architectures for tissue engineering applications | Iwan Zeina, Dietmar W. Hutmacherb,*, Kim Cheng Tanc, Swee Hin Teoh | 2002 | Biomaterials 23 (2002) 1169–1185 | Creation of new materials | mechanics | hive structure | ||||
| 2017 | M16 | Mechanical properties of parts fabricated with inkjet 3D printing through efficient experimental design | J. Mueller a,⁎, K. Shea a, C. Daraio b | 2015 | Materials and Design 86 (2015) 902–912 | mechanical modeling | mechanics | deer | ||||
| 2017 | M17 | Low-cycle fatigue behavior of 3d-printed PLA-based porous scaffolds | F.S. Senatov*, K.V. Niaza, A.A. Stepashkin, S.D. Kaloshkin | 2016 | Composites Part B 97 (2016) 193e200 | mechanical modeling | mechanics | fatigue | ||||
| 2017 | M18 | Mechanical properties of FDM and SLA low-cost 3-D prints | Ksawery Szykiedansa,*, Wojciech Credoa | 2016 | Procedia Engineering 136 ( 2016 ) 257 – 262 | mechanical modeling | mechanics | Low-cost printers | ||||
| 2017 | M19 | Fiber reinforcement during 3D printing | Susanne Christ,Martin Schnabel,Elke Vorndran,Jürgen Groll,Uwe Gbureck | 2015 | Materials Letters139(2015)165–168 | mechanical modeling | mechanics | Additive | ||||
| 2017 | M20 | Effect of layer printing delay on mechanical properties and dimensional accuracy of 3D printed porous prototypes in bone tissue engineering | Arghavan Farzadia,n, Vicknes Waranb, MehranSolati-Hashjina, ZainalAriffAbdulRahmanc, Mitra Asadia, NoorAzuanAbuOsmana | 2015 | Ceramics International41(2015)8320–8330 | mechanical modeling | mechanics | Cooling time, bone, pore | ||||
| 2017 | M21 | Fabrication of imitative cracks by 3D printing for electromagnetic nondestructive testing and evaluations | NoritakaYusa∗, WeixiChen, JingWang, HidetoshiHashizume | 2016 | Case StudiesinNondestructiveTestingandEvaluation5(2016)9–14 | mechanical modeling | mechanics | complementary essay | ||||
| 2017 | M22 | New application of 3D printing method for photostress investigation | Péter Ficzerea *, Lajos Borbásb | 2016 | Materials Today: Proceedings 3 ( 2016 ) 969 – 972 | mechanical modeling | mechanics | complementary essay | ||||
| 2017 | M23 | Caracterización experimental de las constantes elásticas y propiedades mecánicas del ABS en el proceso de impresión 3D | Algarín R. a, Guillen D. b & Fuentes W. c | 2016 | - | mechanical modeling | mechanics | Sure, I can help you with that. Here is the translation of modelacion into English: modeling I have removed the quotation and double quotation marks from the translated value. | ||||
| 2017 | M24 | The effects of moisture and temperature on the mechanical properties of additive manufacturing components: fused deposition modeling | Eunseob Kim, Yong-Jun Shin, Sung-Hoon Ahn | 2016 | Rapid Prototyping Journal, Vol. 22 Issue: 6,pp. 887-894 | mechanical modeling | mechanics | Sure, I can help you with that. Here is the translation of modelacion into English: modeling I have removed the quotation and double quotation marks from the translated value. | ||||
| 2017 | M25 | Preliminary design and analysis of tensile test samples developed by Additive Manufacturing | Wendt, C., Batista, M., Moreno, E., (...), Droste, O., Marcos, M. | 2015 | Procedia Engineering 132, pp. 132-139 | New mechanical tests | mechanics | modeling and testing | ||||
| 2017 | M26 | Mechanical property characterization and simulation of fused deposition modeling Polycarbonate parts | Miquel Domingo-Espin a, Josep M. Puigoriol-Forcada a, Andres-Amador Garcia-Granada a, Jordi Llumà c, Salvador Borros b, Guillermo Reyes a,⇑ | 2015 | Materials & Design 83 (2015) 670–677 | mechanical modeling | simulation and modeling | simulation | ||||
| 2017 | M27 | Modeling and characterization of fused deposition modeling tooling forvacuum assisted resin transfer molding process | H. Li, G. Taylor, V. Bheemreddy, O. Iyibilgin, M. Leu, K. Chandrashekhara | 2015 | Additive Manufacturing 7 (2015) 64–72 | mechanical modeling | simulation and modeling | Thermal efforts | ||||
| 2017 | M28 | Comparative between FEM models for FDM parts and their approach to a real mechanical behaviour | J. Martíneza,*, J.L. Diégueza, E. Aresb, A. Pereirab, P. Hernándezb, J.A. Pérezb | 2013 | Procedia Engineering 63 ( 2013 ) 878 – 884 | mechanical modeling | simulation and modeling | simulation | ||||
| 2017 | M29 | ANALYSIS OF EFFECT OF INTERNAL STRUCTURES ON TENSILE STRENGTH OF THE FDM PARTS | Beulah Mani Paleti1, Karteek Navuri2, Eswara Kumar A.3, Putti Venkata Siva Teja4 | 2017 | International Journal of Pure and Applied Mathematics, Volume 115 No. 6 2017, 123-131 | mechanical modeling | simulation and modeling | simulation | ||||
| 2017 | M30 | EFFECT OF INTERNAL STRUCTURES ON COMPRESSIVE STRENGTH OF THE FDM PARTS | Beulah Mani Paleti1, Karteek Navuri, Eswara Kumar A.3, J. N. Malleswara Rao | 2017 | International Journal of Pure and Applied Mathematics, Volume 115 No. 6 2017, 139-146 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M31 | Studies on Effect of Fused Deposition Modelling Process Parameters on Ultimate Tensile Strength and Dimensional Accuracy of Nylon | C K Basavaraj and M Vishwas | 2016 | IOP Conference Series: Materials Science and Engineering | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M32 | Mechanical behavior of additive manufactured, powder-bed laser-fused materials | Todd M. Mower n, Michael J. Long | 2016 | Materials Science & Engineering A 651 (2016) 198–213 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M33 | Experimental characterization of the tensile strength of ABS parts manufactured by fused deposition modeling process | Kyle Raneya, Eric Lanib, Devi K.Kallac,* | 2017 | Materials Today: Proceedings 4 (2017) 7956–7961 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M34 | Additive manufacturing of PLA structures using fused deposition modelling: Effect of process parameters on mechanical properties and their optimal selection | J.M. Chacón, M.A. Caminero,*, E. García-Plaza, P.J. Núñez | 2017 | Materials and Design 124 (2017) 143–157 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M35 | Experimental characterization and micrography of 3D printed PLA and PLA reinforced with short carbon fibers | Rafael Thiago Luiz Ferreira a, *, Igor Cardoso Amatte a, c, Thiago Assis Dutra a, | 2017 | Composites Part B 124 (2017) 88e100 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M36 | Improving the impact strength of Poly(lactic acid) (PLA) in fused layer modeling (FLM) | Lu Wang a, b, *, William M. Gramlich c, Douglas J. Gardner a, b | 2017 | Polymer 114 (2017) 242e248 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M37 | Experimental investigation of creep deformation of part processed by fused deposition modeling using definitive screening design | Omar Ahmed Mohameda,∗, Syed Hasan Masooda, Jahar Lal Bhowmikb | 2017 | Additive Manufacturing 18 (2017) 164–170 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M38 | Investigation of mechanical anisotropy of the fused filament fabrication process via customized tool path generation | Carsten Koch, Luke Van Hulle∗, Natalie Rudolph | 2017 | Additive Manufacturing 16 (2017) 138–145 | mechanical modeling | mechanics | MODELING AND SIMULATION | ||||
| 2017 | M39 | An insight to the failure of FDM parts under tensile loading: finite element analysis and experimental study | Ashu Garg, Anirban Bhattacharya⁎ | 2017 | International Journal of Mechanical Sciences 120 (2017) 225–236 | mechanical modeling | mechanics | MODELING AND SIMULATION | ||||
| 2017 | M40 | Residual stress measurement in Fused Deposition Modelling parts | Caterina Casavola, Alberto Cazzato, Vincenzo Moramarco*, Giovanni Pappalettera | 2017 | Polymer Testing 58 (2017) 249e255 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M41 | Measurements of the mechanical response of unidirectional 3D-printed PLA | Y. Song, Y. Li, W. Song, K. Yee, K.-Y. Lee, V.L. Tagarielli | 2017 | Materials and Design 123 (2017) 154–164 | mechanical modeling | mechanics | MODELING AND SIMULATION | ||||
| 2017 | M42 | FEM based evaluation of Fused Layer Modelling monolayers in tensile testing | C.WendtaA.P.ValergaaO.DrostebM.BatistaaM.Marcosa | 2017 | Procedia Manufacturing Volume 13, 2017, Pages 916-923 | mechanical modeling | mechanics | MODELING | ||||
| 2017 | M43 | Characterization of Material Behavior of the Fused Deposition Modeling Processed Parts | Madhukar Somireddy and Aleksander Czekanski | 2017 | Volume 2: Additive Manufacturing; Materials ASME 2017 12th International Manufacturing Science and Engineering Conference collocated with the JSME/ASME 2017 6th International Conference on Materials and Processin | mechanical modeling | mechanics | MODELING AND SIMULATION | ||||
| 2017 | M44 | Experimental characterization of the tensile strength of ABS parts manufactured by fused deposition modeling process | Kyle Raneya, Eric Lanib, Devi K.Kallac,* | 2017 | Materials Today: Proceedings 4 (2017) 7956–7961 | mechanical modeling | mechanics | MODELING AND SIMULATION | ||||
| 2017 | M45 | Applications of Fiber-Reinforced Polymers in Additive Manufacturing | Thomas Hofstätter and David B. Pedersen and Guido Tosello and Hans N. Hansen | 2017 | Procedia CIRP 66 ( 2017 ) 312 – 316 | mechanical modeling | mechanics | MODELING AND SIMULATION | ||||
| 2017 | M46 | Fused filament fabrication of fiber-reinforced polymers: A review | Bastian Brenken and Eduardo Barocio and Anthony Favaloro and Vlastimil Kunc and R. Byron Pipes | 2018 | Additive Manufacturing 21 (2018) 1–16 | mechanical modeling | mechanics | MODELING AND SIMULATION | ||||
| 2017 | M47 | Materials for additive manufacturing | David Bourell (2)a,*, Jean Pierre Kruth (1)b, Ming Leu (1)c, Gideon Levy (1)d, David Rosen e, Allison M. Beese f, Adam Clare g | 2017 | CIRP Annals - Manufacturing Technology 66 (2017) 659–681 | Creation of new materials | mechanics | Properties of Materials | ||||
| 2019 | M48 | Interface and performance of 3D printed continuous carbon fiber reinforced PLA composites | Tian, X., Liu, T., Yang, C., Wang, Q., Li, D. | 2016 | Composites Part A: Applied Science and Manufacturing 88, pp. 198-205 | Creation of new materials | mechanics, manufacturing | Additive | ||||
| 2019 | M49 | Single-layer temperature-adjusting transition method to improve the bond strength of 3D-printed PCL/PLA parts | Lin, W., Shen, H., Xu, G., (...), Fu, J., Deng, X. | 2018 | Composites Part A: Applied Science and Manufacturing 115, pp. 22-30 | mechanical modeling | mechanics | temperature | ||||
| 2019 | M50 | 3D printed, bio-inspired prototypes and analytical models for structured suture interfaces with geometrically-tuned deformation and failure behavior | Lin, E., Li, Y., Ortiz, C., Boyce, M.C. | 2014 | Journal of the Mechanics and Physics of Solids 73, pp. 166-182 | mechanical modeling | mechanics | design and shapes | ||||
| 2019 | M51 | Biomimetic staggered composites with highly enhanced energy dissipation: Modeling, 3D printing, and testing | Zhang, P., Heyne, M.A., To, A.C. | 2015 | Journal of the Mechanics and Physics of Solids 83,2677, pp. 285-300 | mechanical modeling | mechanics | multimaterial | ||||
| 2019 | M52 | Mechanical performance of additively-manufactured anisotropic and isotropic smooth shell-lattice materials: Simulations & experiments | Bonatti, C., Mohr, D. | 2019 | Journal of the Mechanics and Physics of Solids 122, pp. 1-26 | mechanical modeling | mechanics | modeling and lattice experiments | ||||
| 2019 | M53 | Preparation and characterization of 3D printed continuous carbon fiber reinforced thermosetting composites | Hao, W., Liu, Y., Zhou, H., Chen, H., Fang, D. | 2018 | Polymer Testing 65, pp. 29-34 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M54 | FDM process parameters influence over the mechanical properties of polymer specimens: A review | Popescu, D., Zapciu, A., Amza, C., Baciu, F., Marinescu, R. | 2018 | Polymer Testing 69, pp. 157-166 | mechanical modeling | mechanics | State of the art, mechanical characterization | ||||
| 2019 | M55 | Residual Stress in Metal Additive Manufacturing | Li, C., Liu, Z.Y., Fang, X.Y., Guo, Y.B. | 2018 | Procedia CIRP 71, pp. 348-353 | mechanical modeling | mechanics | Residual efforts | ||||
| 2019 | M55b | Review of the effect of built orientation on mechanical Properties of metal-plastic composite parts fabricated by Additive Manufacturing Technique | Swapnil Magara, Nitin K. Khedkarb, Satish Kumarc | 2017 | Materials Today: Proceedings 5 (2018) 3926–3935 | mechanical modeling | mechanics | - | ||||
| 2019 | M56 | Evaluation and prediction of the tensile properties of continuous fiber-reinforced 3D printed structures | Melenka, G.W., Cheung, B.K.O., Schofield, J.S., Dawson, M.R., Carey, J.P. | 2016 | Composite Structures 153, pp. 866-875 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M57 | A review on additive manufacturing of polymer-fiber composites | Parandoush, P., Lin, D. | 2017 | Composite Structures 182, pp. 36-53 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M58 | Characterization of 3D printed long fibre reinforced composites | Justo, J., Távara, L., García-Guzmán, L., París, F. | 2018 | Composite Structures 185, pp. 537-548 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M59 | 3D printed continuous fibre reinforced composite corrugated structure | Hou, Z., Tian, X., Zhang, J., Li, D. | 2018 | Composite Structures 184, pp. 1005-1010 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M60 | Orthotropic mechanical properties of fused deposition modelling parts described by classical laminate theory | Casavola, C., Cazzato, A., Moramarco, V., Pappalettere, C. | 2016 | Materials and Design 90, pp. 453-458 | mechanical modeling | mechanics | The translated value of MODELO TEORICO Y EXPERIMENTOS in English is THEORETICAL MODEL AND EXPERIMENTS. | ||||
| 2019 | M61 | Characterization of mechanical properties and fracture mode of additively manufactured carbon fiber and glass fiber reinforced thermoplastics | Goh, G.D., Dikshit, V., Nagalingam, A.P., (...), Wei, J., Yeong, W.Y. | 2018 | Materials and Design 137, pp. 79-89 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M62 | Mechanical properties and deformation behavior of additively manufactured lattice structures of stainless steel | Köhnen, P., Haase, C., Bültmann, J., (...), Schleifenbaum, J.H., Bleck, W. | 2018 | Materials and Design 145, pp. 205-217 | mechanical modeling | mechanics | Sure, I can help you with that. Here is the translation of LATICE into English: Lattice I have removed the quotation and double quotation marks from the translated value. | ||||
| 2019 | M63 | Tensile properties of multi-material interfaces in 3D printed parts | Lumpe, T.S., Mueller, J., Shea, K. | 2019 | Materials and Design 162, pp. 1-9 | mechanical modeling | mechanics | multimaterial | ||||
| 2019 | M64 | Highly oriented carbon fiber-polymer composites via additive manufacturing | Tekinalp, H.L., Kunc, V., Velez-Garcia, G.M., (...), Blue, C.A., Ozcan, S. | 2014 | Composites Science and Technology 105, pp. 144-150 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M65 | 3D-printed PEEK-carbon fiber (CF) composites: Structure and thermal properties | Stepashkin, Chukov, D.I., Senatov, F.S., (...), Korsunsky, A.M., Kaloshkin, S.D. | 2018 | Composites Science and Technology 164, pp. 319-326 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M66 | Rapid prototyping of continuous carbon fiber reinforced polylactic acid composites by 3D printing | Li, N., Li, Y., Liu, S. | 2016 | Journal of Materials Processing Technology 238, pp. 218-225 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M67 | Recycling and remanufacturing of 3D printed continuous carbon fiber reinforced PLA composites | Tian, X., Liu, T., Wang, Q., (...), Li, D., Ziegmann, G. | 2017 | Journal of Cleaner Production 142, pp. 1609-1618 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M68 | Additive manufacturing of carbon fiber reinforced thermoplastic composites using fused deposition modeling | Ning, F., Cong, W., Qiu, J., Wei, J., Wang, S. | 2015 | Composites Part B: Engineering 80, pp. 369-378 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M69 | 3D printing of polymer matrix composites: A review and prospective | Wang, X., Jiang, M., Zhou, Z., Gou, J., Hui, D. | 2017 | Composites Part B: Engineering 110, pp. 442-458 | mechanical modeling | mechanics, manufacturing | Additive | ||||
| 2019 | M70 | Impact damage resistance of 3D printed continuous fibre reinforced thermoplastic composites using fused deposition modelling | Caminero, M.A., Chacón, J.M., García-Moreno, I., Rodríguez, G.P. | 2018 | Composites Part B: Engineering 148, pp. 93-103 | mechanical modeling | mechanics, manufacturing | Additive | ||||
| 2019 | M71 | Mechanical behaviour of ABS: An experimental study using FDM and injection moulding techniques | Dawoud, M., Taha, I., Ebeid, S.J. | 2016 | Journal of Manufacturing Processes | mechanical modeling | mechanics | Comparison processes | ||||
| 2019 | M72 | Mechanical performance of additively manufactured meta-biomaterials | Zadpoor, A.A. | 2019 | Acta Biomaterialia 85, pp. 41-59 | mechanical modeling | mechanics | REVIEW, LATICE | ||||
| 2019 | M73 | Numerical investigation of the mechanical properties of the additive manufactured bone scaffolds fabricated by FDM: The effect of layer penetration and post-heating | Naghieh, S., Karamooz Ravari, M.R., Badrossamay, M., Foroozmehr, E., Kadkhodaei, M. | 2016 | Journal of the Mechanical Behavior of Biomedical Materials 59, pp. 241-250 | mechanical modeling | mechanics, medicine | lattice | ||||
| 2019 | M74 | Selecting process parameters in RepRap additive manufacturing system for PLA scaffolds manufacture | De Ciurana, J., Serenó, L., Vallès, È. | 2013 | Procedia CIRP 5, pp. 152-157 | mechanical modeling | mechanics, medicine, manufacturing | lattice | ||||
| 2019 | M75 | Characterizing the effect of additives to ABS on the mechanical property anisotropy of specimens fabricated by material extrusion 3D printing | Torrado, A.R., Shemelya, C.M., English, J.D., (...), Wicker, R.B., Roberson, D.A. | 2015 | Additive Manufacturing 6, pp. 16-29 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M76 | Comparison of stress concentrator fabrication for 3D printed polymeric izod impact test specimens | David A. Roberson and Angel R. Torrado Perez and Corey M. Shemelya and Armando Rivera and Eric MacDonald and Ryan B. Wicker | 2015 | Additive Manufacturing 7 (2015) 1–11 | mechanical modeling | mechanics | multiprocessing | ||||
| 2019 | M77 | Fabrication of continuous carbon, glass and Kevlar fibre reinforced polymer composites using additive manufacturing | Andrew N. Dickson∗, James N. Barry, Kevin A. McDonnell, Denis P. Dowling | 2017 | Additive Manufacturing 16 (2017) 146–152 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M78 | A survey of finite element analysis of temperature and thermal stress fields in powder bed fusion Additive Manufacturing | Zhibo Luo and Yaoyao Zhao | 2018 | Additive Manufacturing 21 (2018) 318–332 | mechanical modeling | mechanics | thermal efforts | ||||
| 2019 | M79 | Mechanical characterization of 3D-printed polymers | Dizon, J.R.C., Espera, A.H., Chen, Q., Advincula, R.C. | 2018 | Additive Manufacturing 20, pp. 44-67 | mechanical modeling | mechanics | State of the art, mechanical characterization | ||||
| 2019 | M80 | An investigation into 3D printing of fibre reinforced thermoplastic composites | Blok, L.G., Longana, M.L., Yu, H., Woods, B.K.S. | 2018 | Additive Manufacturing 22, pp. 176-186 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M81 | Revealing mechanisms of residual stress development in additive manufacturing via digital image correlation | Bartlett, J.L., Croom, B.P., Burdick, J., Henkel, D., Li, X. | 2018 | Additive Manufacturing 22, pp. 1-12 | mechanical modeling | mechanics | Residual efforts | ||||
| 2019 | M82 | Multi-material 3D printing: The relevance of materials affinity on the boundary interface performance | Lopes, L.R., Silva, A.F., Carneiro, O.S. | 2018 | Additive Manufacturing 23, pp. 45-52 | mechanical modeling | mechanics | multimaterial | ||||
| 2019 | M83 | Mechanical properties of Sn63Pb37 components by fused coating technology | Zhao, G., Wei, Z., Du, J., Geng, R., Xu, S. | 2018 | Additive Manufacturing 22, pp. 388-393 | mechanical modeling | mechanics | coating | ||||
| 2019 | M84 | Influence of printing parameters on the stability of deposited beads in fused filament fabrication of poly(lactic) acid | Bakrani Balani, S., Chabert, F., Nassiet, V., Cantarel, A. | 2019 | Additive Manufacturing 25, pp. 112-121 | mechanical modeling | mechanics | Filament parameters | ||||
| 2019 | M85 | Interlayer fracture toughness of additively manufactured unreinforced and carbon-fiber-reinforced acrylonitrile butadiene styrene | Young, D., Wetmore, N., Czabaj, M. | 2018 | Additive Manufacturing 22, pp. 508-515 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M86 | Development and validation of extrusion deposition additive manufacturing process simulations | Brenken, B., Barocio, E., Favaloro, A., Kunc, V., Pipes, R.B. | 2019 | Additive Manufacturing 25, pp. 218-226 | mechanical modeling | mechanics | simulation process and deformations | ||||
| 2019 | M87 | The influence of forced-air cooling on a 3D printed PLA part manufactured by fused filament fabrication | Lee, C.-Y., Liu, C.-Y. | 2019 | Additive Manufacturing 25, pp. 196-203 | mechanical modeling | mechanics | cooling effect | ||||
| 2019 | M88 | Mechanical properties of hexagonal lattice structures fabricated using continuous liquid interface production additive manufacturing | McGregor, D.J., Tawfick, S., King, W.P. | 2019 | Additive Manufacturing 25, pp. 10-18 | mechanical modeling | mechanics | Lattice | ||||
| 2019 | M89 | Experimental Study on Mechanical Properties of Single- and Dual-material 3D Printed Products | Kim, H., Park, E., Kim, S., (...), Kim, N., Lee, S. | 2017 | Procedia Manufacturing 10, pp. 887-897 | mechanical modeling | mechanics | multimaterial | ||||
| 2019 | M90 | Mechanical strength of chunk-based printed parts for cooperative 3D printing | Poudel, L., Sha, Z., Zhou, W. | 2018 | Procedia Manufacturing 26, pp. 962-972 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2019 | M91 | Biomimetic additive manufactured polymer composites for improved impact resistance | Gu, G.X., Takaffoli, M., Hsieh, A.J., Buehler, M.J. | 2016 | Extreme Mechanics Letters 9, pp. 317-323 | mechanical modeling | mechanics | Additive | ||||
| 2019 | M92 | Strengthening in fracture toughness of a smart material manufactured by 3D printing | Lanzillotti, P., Gardan, J., Makke, A., Recho, N. | 2018 | IFAC-PapersOnLine 51(11), pp. 1353-1358 | mechanical modeling | mechanics | OPTIMIZATION | ||||
| 2019 | M93 | Mechanical properties of 3D printed polymer specimens | V.D. Sagias and K.I. Giannakopoulos and C. Stergiou | 2018 | Procedia Structural Integrity | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2019 | M94 | Mechanical characterization of parts fabricated using fused deposition modeling | Bellini, A., Güçeri, S. | 2003 | Rapid Prototyping Journal 9(4), pp. 252-264 | mechanical modeling | mechanics | simulation | ||||
| 2019 | M95 | Improving the Impact Strength and Heat Resistance of 3D Printed Models: Structure, Property, and Processing Correlationships during Fused Deposition Modeling (FDM) of Poly(Lactic Acid) | Benwood, C., Anstey, A., Andrzejewski, J., Misra, M., Mohanty, A.K. | 2018 | ACS Omega 3(4), pp. 4400-4411 | mechanical modeling | mechanics, manufacturing | multiprocessing | ||||
| 2019 | M96 | Effect of support on printed properties in fused deposition modelling processes | Jiang, J., Lou, J., Hu, G. | 2019 | Virtual and Physical Prototyping | mechanical modeling | mechanics, dimension | support effect | ||||
| 2019 | M97 | Experimental Investigations of Process Parameters Influence on Rheological Behavior and Dynamic Mechanical Properties of FDM Manufactured Parts | Mohamed, O.A., Masood, S.H., Bhowmik, J.L. | 2016 | Materials and Manufacturing Processes 31(15), pp. 1983-1994 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2019 | M98 | Bonding quality and fracture analysis of polyamide 12 parts fabricated by fused deposition modeling | Li, H., Zhang, S., Yi, Z., (...), Guo, J., Xu, G. | 2017 | Rapid Prototyping Journal 23(6), pp. 973-982 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2019 | M99 | Experimental characterization of the mechanical properties of 3D-printed ABS and polycarbonate parts | Cantrell, J.T., Rohde, S., Damiani, D., (...), Kroese, C., Ifju, P.G. | 2017 | Rapid Prototyping Journal 23(4), pp. 811-824 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2019 | M100 | Investigating impact of five build parameters on the maximum flexural force in FDM specimens - A definitive screening design approach | Luzanin, O., Guduric, V., Ristic, I., Muhic, S. | 2017 | Rapid Prototyping Journal 23(6), pp. 1088-1098 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2019 | M101 | Effect of layer orientation on mechanical properties of rapid prototyped samples | Es-Said, O.S., Foyos, J., Noorani, R., (...), Marloth, R., Pregger, B.A. | 2000 | Materials and Manufacturing Processes 15(1), pp. 107-122 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2019 | M102 | State of the art of additive manufacturing: Review for tolerances, mechanical resistance and production costs | Fera, M., Fruggiero, F., Lambiase, A., Macchiaroli, R. | 2016 | Cogent Engineering 3(1), pp. 1261503 | mechanical modeling | mechanics, dimension, cost | State of the art, mechanical characterization | ||||
| 2019 | M103 | Impact of fused deposition modeling (FDM) process parameters on strength of built parts using Taguchi’s design of experiments | Zaman, U.K., Boesch, E., Siadat, A., Rivette, M., Baqai, A.A. | 2018 | International Journal of Advanced Manufacturing Technology | mechanical modeling | mechanics | Mechanical characterization, time | ||||
| 2021 | M104 | 3D Printing of Continuous Carbon Fibre Reinforced Thermo-Plastic ({CFRTP}) Tensile Test Specimens | Frank Van Der Klift and Yoichiro Koga and Akira Todoroki and Masahito Ueda and Yoshiyasu Hirano and Ryosuke Matsuzaki | 2016 | Journal Article published 2016 in Open Journal of Composite Materials volume 06 issue 01 on pages 18 to 27 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M105 | Structural characteristics of fused deposition modeling polycarbonate material | Walter Castro Smith and Richard W. Dean | 2013 | Journal Article published Dec 2013 in Polymer Testing volume 32 issue 8 on pages 1306 to 1312 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M106 | Additive manufacturing of {PLA} structures using fused deposition modelling: Effect of process parameters on mechanical properties and~their optimal selection | J.M. Chacón, M.A. Caminero, E. García-Plaza, P.J. Núñez | 2017 | Journal Article published Jun 2017 in Materials & Design volume 124 on pages 143 to 157 | mechanical modeling/optimization | mechanics, optimization | Mechanical characterization, strength optimization, time optimization. | ||||
| 2021 | M107 | A study of creep in polycarbonate fused deposition modelling parts | Antonio G. Salazar-Martín and Marco A. Pérez and Andrés-Amador García-Granada and Guillermo Reyes and Josep M. Puigoriol-Forcada | 2018 | Materials & Design Volume 141, 5 March 2018, Pages 414-425 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M108 | Evaluation of the influence of build and print orientations of unmanned aerial vehicle parts fabricated using fused deposition modeling process | Suraj Ravindrababu and Yunus Govdeli and Zhuo Wei Wong and Erdal Kayacan | 2018 | Journal of Manufacturing Processes Volume 34, Part A, August 2018, Pages 659-666 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M109 | Quality improvement of FDM parts by parameter optimization | Knoop,F. and Kloke,A. and Schoeppner,V. | 2017 | AIP Conference Proceedings 1914, 190001 (2017); | mechanical modeling/optimization | mechanics, optimization | Mechanical characterization, strength optimization, time optimization. | ||||
| 2021 | M110 | A comprehensive review of selected biological armor systems – From structure-function to bio-mimetic techniques | Tu Van Le and Abdallah Ghazlan and Tuan Ngo and Tuan Nguyen and Alex Remennikov | 2019 | Composite Structures 225 (2019) 111172 | mechanical modeling | mechanics | Structures in nature, literature review | ||||
| 2021 | M111 | Materials with enhanced adhesive properties based on acrylonitrile-butadiene-styrene (ABS)/thermoplastic polyurethane (TPU) blends for fused filament fabrication (FFF) | A.S. de León and A. Domínguez-Calvo and S.I. Molina | 2019 | Materials and Design 182 (2019) 108044 | mechanical modeling | mechanics | Mechanical characterization, multimaterial | ||||
| 2021 | M112 | Ultimate Tensile Strength in Fused Deposition Modeling Considering Process Parameters of Flow Rate and Printing Head Speed | Tao Hou, Tingting Huang, Fuqiang Sun, Shanggang Wang | 2018 | Proceedings Article published Oct 2018 in 2018 12th International Conference on Reliability, Maintainability, and Safety (ICRMS) | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M113 | An experimental study on interfacial fracture toughness of 3-D printed ABS/CF-PLA composite under mode I, II, and mixed-mode loading | Abdul Samad Khan and Aaqib Ali and Ghulam Hussain and Muhammad Ilyas | 2019 | Journal of Thermoplastic Composite Materials 1–24 2019 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M114 | Mechanical properties of 3D parts fabricated by fused deposition modeling: Effect of various fillers in polylactide | Xia Gao and Daijun Zhang and Shunxin Qi and Xiangning Wen and Yunlan Su | 2019 | Journal Article published 15 Aug 2019 in Journal of Applied Polymer Science volume 136 issue 31 on page 47824 | mechanical modeling | mechanics | Mechanical characterization, material additive, process chain | ||||
| 2021 | M115 | Experimental investigation on flexural properties of {FDM} processed Nylon 12 parts using {RSM} | Salam Nori Kamoona and Syed Hasan Masood and Omar Ahmed Mohamed | 2018 | Journal Article published Jun 2018 in IOP Conference Series: Materials Science and Engineering volume 377 on page 012137 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M116 | MECHANICAL PROPERTIES OF PRODUCTS MADE OF ABS WITH RESPECT TO INDIVIDUALITY OF FDM PRODUCTION PROCESSES | Martin Seidl and Jiri Safka and Jiri Bobek and Lubos Behalek and Jiri Habr | 2017 | Journal Article published 8 Feb 2017 in MM Science Journal volume 2017 issue 01 on pages 1748 to 1751 | mechanical modeling | mechanics | Mechanical characterization, printer comparison. | ||||
| 2021 | M117 | The influence of slicing parameters on the multi-material adhesion mechanisms of FDM printed parts: an exploratory study | Francesco Tamburrino and Serena Graziosi and Monica Bordegoni | 2019 | VIRTUAL AND PHYSICAL PROTOTYPING 2019, VOL. 14, NO. 4, 316–332 | mechanical modeling | mechanics | Mechanical characterization, multimaterial | ||||
| 2021 | M118 | Design considerations and modeling of fiber reinforced 3D printed parts | Nekoda {van de Werken} and Joel Hurley and Pouria Khanbolouki and Ali N. Sarvestani and Ali Y. Tamijani and Mehran Tehrani | 2019 | Composites Part B: Engineering Volume 160, 1 March 2019, Pages 684-692 | mechanical modeling | mechanics | Mechanical characterization, multimaterial | ||||
| 2021 | M119 | Selecting Process Parameters in {RepRap} Additive Manufacturing System for {PLA} Scaffolds Manufacture | Joaquim de Ciurana, Lídia Serenóa, Èlia Vallès | 2013 | Journal Article published 2013 in Procedia CIRP volume 5 on pages 152 to 157 | mechanical modeling, medicine | mechanics, medicine | Mechanical characterization, scaffolding for tissue. | ||||
| 2021 | M120 | Comparison of Numerical Methods for Fluid-Structure Interaction Simulation of Fused Deposition Modeled Nylon Components | Sumair F. Sunny and Glenn H. Gleason and Arif S. Malik | 2019 | Journal Article published 2019 in Procedia Manufacturing volume 34 on pages 516 to 527 | mechanical modeling, dimensional modeling | mechanics, dimension | MODELING AND SIMULATION | ||||
| 2021 | M121 | Advances in fused deposition modeling of discontinuous fiber/polymer composites | Chao Hu and Qing-Hua Qin | 2020 | Current Opinion in Solid State and Materials Science Volume 24, Issue 5, October 2020, 100867 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial | ||||
| 2021 | M122 | Additive manufacturing of mechanochromic polycaprolactone on entry-level systems | Gregory I. Peterson, Mete Yurtoglu, Michael B Larsen, Stephen L. Craig, Mark A. Ganter, Duane W. Storti, Andrew J. Boydston | 2015 | Journal Article published 17 Aug 2015 in Rapid Prototyping Journal volume 21 issue 5 on pages 520 to 527 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, electrical, chemical. | ||||
| 2021 | M123 | Effect of layer thickness on irreversible thermal expansion and interlayer strength in fused deposition modeling | Anthony A. D’Amico, Analise Debaie, Amy M. Peterson | 2017 | Journal Article published 22 Aug 2017 in Rapid Prototyping Journal volume 23 issue 5 on pages 943 to 953 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization | ||||
| 2021 | M124 | Investigation on the tribological behavior and wear mechanism of parts processed by fused deposition additive manufacturing process | Omar Ahmed Mohamed and Syed Hasan Masood and Jahar Lal Bhowmik and Anthony E. Somers | 2017 | Journal of Manufacturing Processes 29 (2017) 149–159 | mechanical modeling | mechanics | Mechanical characterization, wear, friction | ||||
| 2021 | M125 | Analysis of wear behavior of additively manufactured PC-ABS parts | Omar Ahmed Mohamed and Syed Hasan Masood and Jahar Lal Bhowmik | 2018 | Materials Letters Volume 230, 1 November 2018, Pages 261-265 | mechanical modeling | mechanics | Mechanical characterization, wear, friction | ||||
| 2021 | M126 | A study on extruded filament bonding in fused filament fabrication | Ana Elisa Costa and Alexandre Ferreira da Silva and Olga Sousa Carneiro | 2019 | Journal Article published 8 Apr 2019 in Rapid Prototyping Journal volume 25 issue 3 on pages 555 to 565 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M127 | Anisotropic material properties of fused deposition modeling {ABS} | Sung‐Hoon Ahn, Michael Montero, Dan Odell, Shad Roundy, Paul K. Wright | 2002 | Journal Article published Oct 2002 in Rapid Prototyping Journal volume 8 issue 4 on pages 248 to 257 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M128 | Mechanical properties of commercial rapid prototyping materials | Jaroslaw Kotlinski | 2014 | Journal Article published 20 Oct 2014 in Rapid Prototyping Journal volume 20 issue 6 on pages 499 to 510 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M129 | CARACTERIZACIÓN DE MATERIALES TERMOPLÁSTICOS DE ABS Y PLA SEMI - RÍGIDO IMPRESOS EN 3D CON CINCO MALLADOS INTERNOS DIFERENTES | JAIME VINICIO MOLINA OSEJOS | 2016 | ESCUELA POLITÉCNICA NACIONAL, FACULTAD DE INGENIERÍA MECÁNICA (TESIS DE MAESTRIA) | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M130 | Dimensional considerations on the mechanical properties of 3D printed polymer parts | Nabila Elmrabet and Petros Siegkas | 2020 | Polymer Testing Volume 90, October 2020, 106656 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M131 | Comparison of tribological behavior of nylon aramid polymer composite fabricated by fused deposition modeling and injection molding process | J Nagendra, M S Ganesha Prasad, S Shashank, Syed Md. Ali | 2018 | Int. J. Mech. Mech. Eng. Technol Volume 9, Issue 13, December 2018 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M132 | Tribological properties of 3D-printed pin with internal structure formation under dry sliding conditions | Tahir, Noor Ayuma Mat and Azmi, Muhamad Syafwan and Abdollah, Mohd Fadzli Bin and Ramli, Faiz Redza and Amiruddin, Hilmi and Tokoroyama, Takayuki and Umehara, Noritsugu | 2018 | Proceedings of Mechanical Engineering Research Day 2018, pp. 260-261, May 2018 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M133 | Predicting strength of additively manufactured thermoplastic polymer parts produced using material extrusion | Joseph Bartolai and Timothy W. Simpson and Renxuan Xie | 2018 | Journal Article published 12 Mar 2018 in Rapid Prototyping Journal volume 24 issue 2 on pages 321 to 332 | mechanical modeling, failure theory | mechanics | Mechanical characterization, simulation | ||||
| 2021 | M134 | Fused filament fabrication of polymer materials: A review of interlayer bond | Xia Gao and Shunxin Qi and Xiao Kuang and Yunlan Su and Jing Li and Dujin Wang | 2021 | Journal Article published 9 Apr 2018 in Rapid Prototyping Journal volume 24 issue 3 on pages 645 to 669 | mechanical modeling | mechanics | Mechanical characterization, state of the art | ||||
| 2021 | M135 | A critical review on 3D printed continuous fiber-reinforced composites: History, mechanism, materials and properties | S M Fijul Kabir and Kavita Mathur and Abdel-Fattah M. Seyam | 2020 | Composite Structures Volume 232, 15 January 2020, 111476 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess. | ||||
| 2021 | M136 | Friction and hardness characteristics of FDM-printed plastic materials | Sahar Zhiani Hervan Zeynep Parlar Vedat Temiz Atakan Altınkaynak | 2018 | 21st International Research/Expert Conference ”Trends in the Development of Machinery and Associated Technology” TMT 2018, Karlovy Vary, Czech Republic, 18th – 22nd September, 2018 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M137 | Anisotropic damage inferred to 3D printed polymers using fused deposition modelling and subject to severe compression | Sofiane Guessasma and Sofiane Belhabib and Hedi Nouri and Omar {Ben Hassana} | 2016 | European Polymer Journal Volume 85, December 2016, Pages 324-340 | mechanical modeling | mechanics | Mechanical characterization, simulation | ||||
| 2021 | M138 | Comparision of tribological behaviour for parts fabricated through fused deposition modelling (FDM) process on abs and 20% carbon fibre PLA | R. Srinivasan and B. Suresh Babu and V. Udhaya Rani and M. Suganthi and R. Dheenasagar | 2020 | Materials Today: Proceedings, Volume 27, Part 2, 2020, Pages 1780-1786 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M139 | Influential analysis of fused deposition modeling process parameters on the wear behaviour of ABS parts | R. Srinivasan and R. Rathish and P.R. Sivaraman and Adwaith Pramod and G. Shivaganesh | 2020 | Materials Today: Proceedings, Volume 27, Part 2, 2020, Pages 1869-1876 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M140 | Influence of fused deposition modelling process parameters on wear strength of carbon fibre PLA | R. Srinivasan and N. Aravindkumar and S. {Aravind Krishna} and S. Aadhishwaran and John George | 2020 | Materials Today: Proceedings,Volume 27, Part 2, 2020, Pages 1794-1800 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M141 | Influence of fused deposition modeling process parameters on the mechanical properties of PETG parts | R. Srinivasan and P. Prathap and Asrith Raj and S. {Aswinth Kannan} and V. Deepak | 2020 | Materials Today: Proceedings 27 (2020) 1877–1883 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M142 | Characterization of process–deformation/damage property relationship of fused deposition modeling (FDM) 3D-printed specimens | Tomas {Webbe Kerekes} and Hyoungjun Lim and Woong Yeol Joe and Gun Jin Yun | 2019 | Additive Manufacturing 25 (2019) 532–544 | mechanical modeling | mechanics | Mechanical characterization, simulation | ||||
| 2021 | M143 | Structural performance of 3D-printed composites under various loads and environmental conditions | Mohammad Reza Khosravani and Ali Zolfagharian and Matt Jennings and Tamara Reinicke | 2020 | Polymer Testing 91 (2020) 106770 | mechanical modeling, failure theory | mechanics, failure theory | Mechanical characterization, failure theory | ||||
| 2021 | M144 | Analysis of the influence of the variables of the Fused Deposition Modeling (FDM) process on the mechanical properties of a carbon fiber-reinforced polyamide | Elena Verdejo de Toro, Juana Coello Sobrino, Alberto Matínez Martínez, Valentín Miguel Eguía | 2019 | Journal Article published 2019 in Procedia Manufacturing volume 41 on pages 731 to 738 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M145 | A physical investigation of wear and thermal characteristics of 3D printed nylon spur gears | Ye Zhang and Chris Purssell and Ken Mao and Simon Leigh | 2020 | Tribology International Volume 141, January 2020, 105953 | mechanical modeling, fatigue modeling | mechanics, fatigue | Mechanical characterization, fatigue in polymers | ||||
| 2021 | M146 | Mechanical structural design based on additive manufacturing and internal reinforcement | João Fiore Parreira Lovo and Italo Leite de Camargo and Luis Antonio Oliveira Araujo and Carlos Alberto Fortulan | 2020 | Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science, volume 234, number 2, pages 417-426 | mechanical modeling, manufacturing | mechanics, manufacturing | Mechanical characterization, process chain, multimaterial, multiprocess, simulation. | ||||
| 2021 | M147 | Joining of ABS parts built by material extrusion: Analysis of strength and fracture behavior | Bitthal Saraf and Ashu Garg and Suman Saurav and Anirban Bhattacharya | 2020 | CIRP Journal of Manufacturing Science and Technology | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess. | ||||
| 2021 | M148 | Increased fracture toughness of additively manufactured semi-crystalline thermoplastics via thermal annealing | Kevin R. Hart and Ryan M. Dunn and Eric D. Wetzel | 2020 | Polymer Volume 211, 21 December 2020, 123091 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess. | ||||
| 2021 | M149 | Interface geometries in 3D multi-material prints by fused filament fabrication | Micaela Ribeiro and Olga Sousa Carneiro and Alexandre Ferreira da Silva | 2019 | Rapid Prototyping Journal volume 25 issue 1 on pages 38 to 46 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess. | ||||
| 2021 | M150 | Analysis of bonding methods for FDM-manufactured parts | Espalin, D and Arcaute, K and Anchondo, E and Adame, A and Medina, F and Winker, R and Hoppe, T and Wicker, R | 2010 | 21st Annual International Solid Freeform Fabrication Symposium-An Additive Manufacturing Conference | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess, glue. | ||||
| 2021 | M151 | Adhesive bonding of FDM-manufactured parts made of ULTEM 9085 considering surface treatment, surface structure, and joint design | Franziska Bürenhaus, Elmar Moritzer, André Hirsch | 2019 | Welding in the World volume 63 issue 6 on pages 1819 to 1832 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess, glue. | ||||
| 2021 | M152 | Characterization of the Mechanical Properties of FFF Structures and Materials: A Review on the Experimental, Computational and Theoretical Approaches | Enrique Cuan-Urquizo, Eduardo Barocio, Viridiana Tejada-Ortigoza, R. Pipes, Ciro Rodriguez, Armando Roman-Flores | 2019 | Materials volume 12 issue 6 on page 895 | mechanical modeling | mechanics | Mechanical characterization, state of the art | ||||
| 2021 | M153 | Failure Analysis and Mechanical Characterization of 3D Printed ABS With Respect to Layer Thickness and Orientation | Behzad Rankouhi, Sina Javadpour, Fereidoon Delfanian, Todd Letcher | 2016 | Journal of Failure Analysis and Prevention volume 16 issue 3 on pages 467 to 481 | mechanical modeling, failure theory | mechanics | Mechanical characterization, failure theory | ||||
| 2021 | M154 | Fractographic analysis of tensile failure of acrylonitrile-butadiene-styrene fabricated by fused deposition modeling | Jaret C. Riddick and Mulugeta A. Haile and Ray Von Wahlde and Daniel P. Cole and Oluwakayode Bamiduro and Terrence E. Johnson | 2016 | Additive Manufacturing Volume 11, July 2016, Pages 49-59 | mechanical modeling, failure theory | mechanics | Mechanical characterization, failure theory | ||||
| 2021 | M155 | Mechanical, thermal and melt flow of aluminum-reinforced PA6/ABS blend feedstock filament for fused deposition modeling | Rupinder Singh, Ranvijay Kumar, IPS Ahuja | 2018 | Rapid Prototyping Journal volume 24 issue 9 on pages 1455 to 1468 | mechanical modeling | mechanics | Mechanical characterization, multimaterial, additive. | ||||
| 2021 | M156 | Investigations on 3D printed thermosetting and ceramic-reinforced recycled thermoplastic-based functional prototypes | Rupinder Singh, Ranvijay Kumar, Inderpreet Singh | 2019 | Journal of Thermoplastic Composite Materials on page 089270571986462 | mechanical modeling | mechanics | Mechanical characterization, multimaterial, additive. | ||||
| 2021 | M157 | Mechanical and morphological investigations of 3D printed recycled ABS reinforced with bakelite–SiC–Al2O3 | Rupinder Singh, Inderpreet Singh, Ranvijay Kumar | 2019 | Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science volume 233 issue 17 on pages 5933 to 5944 | mechanical modeling | mechanics | Mechanical characterization, multimaterial, additive. | ||||
| 2021 | M158 | ESTUDIO EXPERIMENTAL Y OPTIMIZACIÓN DE JUNTAS PEGADAS DE PIEZAS IMPRESAS EN 3D, CON INTERFAZ DE SUPERFICIE ENTRELAZADA | ERWIN ALFREDO MOLINO ALVAREZ SERGIO ANDRES QUINTANA GONZALEZ | 2019 | UNIVERSIDAD DEL ATLÁNTICO FACULTAD DE INGENIERÍA PROGRAMA DE INGENIERÍA MECÁNICA | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess, glue. | ||||
| 2021 | M159 | CARACTERIZACIÓN DE PROBETAS FABRICADAS CON POLICARBONATO POR EL MODELADO POR DEPOSICIÓN FUNDIDA (FDM) | CHRISTIAN GUTIÉRREZ VILLADIEGO, JOSÉ MARÚN ROCA | 2020 | UNIVERSIDAD DEL ATLÁNTICO FACULTAD DE INGENIERÍA PROGRAMA DE INGENIERÍA MECÁNICA | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M160 | CARACTERIZACIÓN MECÁNICA DE PROBETAS DE POLIETILENO TEREPHTHALATE CON GLICOL IMPRESAS EN 3D MEDIANTE EL MÉTODO DE MODELADO POR DEPOSICIÓN FUNDIDA | DARIO LUIS CASTRO ESCORCIA, EDEL CASTAÑO LOPEZ | 2021 | UNIVERSIDAD DEL ATLÁNTICO FACULTAD DE INGENIERÍA PROGRAMA DE INGENIERÍA MECÁNICA | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M161 | Caracterización mecánica de probetas fabricadas con Poliuretano termoplástico (TPU) por el proceso de Modelado de Deposición Fundida (FDM) | Martínez Pedraza Héctor Julio, Rizo Pacheco Adrian Josue | 2021 | UNIVERSIDAD DEL ATLÁNTICO FACULTAD DE INGENIERÍA PROGRAMA DE INGENIERÍA MECÁNICA | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M162 | Experimental study of resin coating to improve the impact strength of fused filament fabrication process pieces | Luis Lisandro López Taborda, Eduar Pérez, Daniel Quintero, José Fernando Noguera Polania, Habib Zambrano Rodriguez, Heriberto Maury, Ivan E. Esparragoza | 2021 | Journal Article published 1 Mar 2021 in Rapid Prototyping Journal volume ahead-of-print issue ahead-of-print | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess, resin. | ||||
| 2021 | M163 | Enhancing durability of 3D printed polymer structures by metallization | Arash Afshar and Dorina Mihut | 2020 | Journal of Materials Science & Technology 53 (2020) 185–191 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, process chain, multiprocess, metallization. | ||||
| 2021 | M164 | Mechanical evaluation of polymeric filaments and their corresponding 3D printed samples | A.M. Oviedo and A.H. Puente and C. Bernal and E. Pérez | 2020 | Polymer Testing 88 (2020) 106561 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial | ||||
| 2021 | M165 | Mechanical characterization of functionally graded materials produced by the fused filament fabrication process | Seymur Hasanov and Ankit Gupta and Aslan Nasirov and Ismail Fidan | 2020 | Journal of Manufacturing Processes 58 (2020) 923–935 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, simulation | ||||
| 2021 | M166 | Process-structure-property effects on ABS bond strength in fused filament fabrication | A.C. Abbott and G.P. Tandon and R.L. Bradford and H. Koerner and J.W. Baur | 2018 | Additive Manufacturing 19 (2018) 29–38 | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M167 | Nonisothermal welding in fused filament fabrication | Keith Coasey and Kevin R. Hart and Eric Wetzel and David Edwards and Michael E. Mackay | 2020 | Additive Manufacturing 33 (2020) 101140 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multiprocess, annealing, analytical modeling | ||||
| 2021 | M168 | Optimising Process Parameters of Fused Filament Fabrication to Achieve Optimum Tensile Strength | Nawaharsh Weake and Meena Pant and Ankita Sheroan and Abid Haleem and Harish Kumar | 2020 | Procedia Manufacturing 51 (2020) 704–709 | mechanical modeling, Optimization | mechanics, optimization | Mechanical characterization, simulation, optimization | ||||
| 2021 | M169 | Anisotropic material properties of fused deposition modeling ABS | Sung-Hoon Ahn and Michael Montero and Dan Odell and Shad Roundy and Paul K. Wright | 2002 | Rapid Prototyping Journal | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M170 | Fracture Surface Analysis of 3D-Printed Tensile Specimens of Novel ABS-Based Materials | Angel R. Torrado Perez, David A. Roberson, Ryan B. Wicker | 2014 | Journal of Failure Analysis and Prevention volume 14 issue 3 on pages 343 to 353 | Mechanical modeling, Manufacturing process cases (general and specific) | mechanics, manufacturing | Mechanical characterization, multimaterial, simulation | ||||
| 2021 | M171 | Anisotropic Mechanical Properties of ABS Parts Fabricated by Fused Deposition Modelling | Constance Ziemian, Mala Sharma, Sophia Ziemi | 2012 | Book Chapter published 11 Apr 2012 in Mechanical Engineering | mechanical modeling | mechanics | Mechanical characterization | ||||
| 2021 | M172 | ABSplus-P430 PRODUCTION-GRADE THERMOPLASTIC FOR 3D PRINTERS | STRATASYS | 2017 | mechanical modeling | mechanics | Mechanical characterization | |||||
| 2021 | M173 | Polymer additive manufacturing of ABS structure: Influence of printing direction on mechanical properties | H. Ramezani Dana, F. Barbe, L. Delbreilh, M. Ben Azzouna, A. Guillet, T. Breteau | 2019 | Journal of Manufacturing Processes 44 (2019) 288–298 | mechanical modeling | mechanics | Mechanical characterization | ||||
| M174 | Improved design of fused deposition modeling equipment for 3D printing of high-performance PEEK parts | Bin Hu, Xianbao Duan, Zehua Xing, Ziyou Xu, Chun Du, Huamin Zhou, Rong Chen, Bin Shan | 2019 | Mechanics of Materials 137 (2019) 103139 | mechanical modeling | mechanics | Mechanical characterization and simulation | |||||
| M175 | Mechanical properties of fused filament fabricated PEEK for biomedical applications depending on additive manufacturing parameters | Yiqiao Wang, Wolf-Dieter Müller, Adam Rumjahn, Franziska Schmidt, Andreas Dominik Schwitalla | 2021 | journal of the mechanical behavior of biomedical materials 115 (2021) 104250 | mechanical modeling | mechanics | Mechanical characterization | |||||
| M176 | Screw extrusion-based additive manufacturing of PEEK | Jian-Wei Tseng, Chao-Yuan Liu, Yi-Kuang Yen, Johannes Belkner, Tobias Bremicker,Bernard Haochih Liu, Ta-Ju Sun, An-BangWang | 2018 | Materials and Design 140 (2018) 209–221 | mechanical modeling | mechanics | Mechanical characterization | |||||
| M177 | Performance of biocompatible PEEK processed by fused deposition additive manufacturing | M.F. Arif, S. Kumar, K.M. Varadarajan, W.J. Cantwell | 2018 | Materials and Design 146 (2018) 249–259 | mechanical modeling | mechanics | Mechanical characterization | |||||
| M178 | Effects of printing parameters of fused deposition modeling on mechanical properties, surface quality, and microstructure of PEEK | Peng Wang, Bin Zou, Hongchuan Xiao, Shouling Ding, Chuanzhen Huang | 2019 | Journal of Materials Processing Tech. 271 (2019) 62–74 | mechanical modeling | mechanics | Mechanical characterization and simulation | |||||
| M179 | 3D printing of PEEK and its composite to increase biointerfaces as a biomedical material- A review | Bankole I. Oladapo, S. Abolfazl Zahedi, Sikiru O. Ismail, Francis T. Omigbodun | 2021 | Colloids and Surfaces B: Biointerfaces 203 (2021) 111726 | mechanical modeling/ medical applications | mechanics / medicine | Mechanical characterization, state of the art, medical applications. | |||||
| M180 | Additive layer manufacturing of poly (ether ether ketone) via FDM | Marianna Rinaldi, Tommaso Ghidini, Federico Cecchinia, Ana Brandao, Francesca Nannia | 2018 | Composites Part B 145 (2018) 162–172 | mechanical modeling | mechanics | Mechanical characterization | |||||
| 2017 | O1 | An optimization approach for components built by fused deposition modeling with parametric internal structures | L. Villalpandoa, H. Eiliata, R. J. Urbanicb* | 2014 | Procedia CIRP 17 ( 2014 ) 800 – 805 | optimization | optimization | Internal structure, mechanical property (AG) | ||||
| 2017 | O2 | Medial axis tree—an internal supporting structure for 3D printing | XiaolongZhanga,∗, YangXiaa,b,∗, JiayeWangc, ZhouwangYangd, ChangheTuc, WenpingWanga | 2015 | ComputerAidedGeometricDesign35–36(2015)149–162 | optimization | optimization | Effort, weight | ||||
| 2017 | O3 | Optimization of fused deposition modeling process using teachinglearning- based optimization algorithm | R. Venkata Rao *, Dhiraj P. Rai | 2016 | Engineering Science and Technology, an International Journal 19 (2016) 587–603 | optimization | optimization | mechanical resistance, dimensional precision, volumetric shrinkage (learning) | ||||
| 2017 | O4 | Optimization of fused deposition modeling process parameters for dimensional accuracy using I-optimality criterion | Omar Ahmed Mohamed a,⇑, Syed Hasan Masood a, Jahar Lal Bhowmik | 2016 | Measurement 81 (2016) 174–196 | optimization | optimization | Dimensional precision (Optimal Criterion I) | ||||
| 2017 | O5 | Multi-criteriaselection of structural adhesives to bond ABS parts obtained by rapid prototyping | Jose´ M. Arenas n, CristinaAlı´a, FernandoBlaya,AlfredoSanz | 2012 | International Journal of Adhesion & Adhesives 33(2012)67–74 | optimization | optimization | Ensemble with glue (AHP) | ||||
| 2017 | O6 | Orientation analysis of 3D objects toward minimal support volume in 3D-printing | Ben Ezair n, FadyMassarwi,GershonElber | 2015 | Computers &Graphics51(2015)117–124 | optimization | optimization | volume piece and support depending on the orientation | ||||
| 2017 | O7 | Optimization of the printing parameters affecting dimensionalaccuracy and internal cavity for HIPS material used in fuseddeposition modeling processes | Mahdi Kaveh∗,1, Mohsen Badrossamay, Ehsan Foroozmehr, Ardeshir Hemasian Etefagh1 | 2015 | Journal of Materials Processing Technology 226 (2015) 280–286 | optimization | optimization | cavity accuracy | ||||
| 2017 | O8 | Slice coherence in a query-based architecture for 3D heterogeneous printing | Ulas Yamana,b,∗, Nabeel Butt a, Elisha Sacks a, Christoph Hoffmanna | 2016 | Computer-Aided Design 75–76 (2016) 27–38 | optimization | optimization | Cells, time and material | ||||
| 2017 | O9 | Topology optimization for fused deposition modeling process | R. Rezaie, M. Badrossamay*, A. Ghaie, H. Moosavi | 2013 | Procedia CIRP 6 ( 2013 ) 521 – 526 | optimization | optimization | topological | ||||
| 2017 | O10 | Printing 3D objects with interlocking parts | PengSonga,∗, ZhongqiFub, LigangLiub, Chi-WingFuc | 2015 | ComputerAidedGeometricDesign35–36(2015)137–148 | optimization | optimization | Easily assemble, and rigid. | ||||
| 2017 | O11 | Modeling and evaluation of curved layer fused deposition | Sarat Singamnenia,∗, Asimava Roychoudhuryb, Olaf Diegela, Bin Huanga | 2012 | Journal of Materials Processing Technology 212 (2012) 27– 35 | optimization | optimization | curved pieces | ||||
| 2017 | O12 | Real time adaptive slicing for fused deposition modelling | P.M. Pandey, N.V. Reddy ∗, S.G. Dhande 1 | 2003 | International Journal of Machine Tools & Manufacture 43 (2003) 61–71 | optimization | optimization | finished | ||||
| 2017 | O13 | A novel approach to improvemechanical properties of parts fabricated by fused deposition modeling | Jianlei Wang a,c, Hongmei Xie a, ZixiangWeng a,c, T. Senthil a, Lixin Wua,b,⁎ | 2016 | Materials and Design 105 (2016) 152–159 | optimization | optimization | Additive | ||||
| 2017 | O14 | Optimization of a heated platform based on statistical annealing of critical design parameters in a 3D printing application | Andrew Rictora, Bryan Rileyb, PhD0F* | 2016 | Procedia Computer Science 83 ( 2016 ) 712 – 716 | optimization | optimization | Machine (economic data) | ||||
| 2017 | O15 | Optimum part deposition orientation in fused deposition modeling | K. Thrimurthulu a, Pulak M. Pandey b, N. Venkata Reddy a, | 2004 | International Journal of Machine Tools & Manufacture 44 (2004) 585–594 | optimization | optimization | orientation | ||||
| 2017 | O16 | Mathematical modeling and FDM process parameters optimization using response surface methodology based on Q-optimal design | Omar Ahmed Mohamed a , ∗, Syed Hasan Masood a , JaharLal Bhowmik b | 2016 | Applied Mathematical Modelling 0 0 0 (2016) 1–22 | optimization | optimization | flexion module, construction time | ||||
| 2017 | O17 | Comparative evaluation of optimization algorithms at training of genetic programming for tensile strength prediction of FDM processed part | Biranchi Narayan Panda, M. V. A Raju Bahubalendruni, bibhuti | 2014 | Procedia Materials Science 5 ( 2014 ) 2250 – 2257 | optimization | optimization | tension resistance | ||||
| 2017 | O18 | Study of compression properties of topologically optimized FDM made structured parts | L.M. Galantucci (1)*, F. Lavecchia, G. Percoco | 2008 | CIRP Annals - Manufacturing Technology 57 (2008) 243–246 | optimization | optimization | topological, compression | ||||
| 2017 | O19 | Integrated design of cellular composites using a level-set topology optimization method | Hao Lia,b, Zhen Luob, Nong Zhangb, Liang Gaoa,∗, Terry Brownb a | 2016 | Comput. Methods Appl. Mech. Engrg. 309 (2016) 453–475 | optimization | optimization | cell phone, topology | ||||
| 2017 | O20 | Optimizing the rapid prototyping process by integrating the Taguchi method with the Gray relational analysis | Che Chung Wang, Ta‐Wei Lin, Shr‐Shiung Hu, | 2007 | Rapid Prototyping Journal, Vol. 13 Issue: 5,pp. 304-315, | optimization | optimization | Experimental optimization | ||||
| 2017 | O21 | Revolution of 3D printing technology and application of Six Sigma methodologies to optimize the output quality characteristics | Chen, J.C., Gabriel, V.S. | 2016 | Proceedings of the IEEE International Conference on Industrial Technology 2016-May,7474872, pp. 904-909 | optimization | optimization | Experimental optimization/quality optimization/Six Sigma | ||||
| 2017 | O22 | Topology optimization and additive manufacturing: Comparison of conception methods using industrial codes | Saadlaoui, Y., Milan, J.-L., Rossi, J.-M., Chabrand, P. | 2017 | Journal of Manufacturing Systems 43, pp. 178-186 | optimization | optimization | State of the art (commercial codes) | ||||
| 2017 | O23 | Studies on Optimizing Process Parameters of Fused Deposition Modelling Technology for ABS | Vishwas.M,a* Basavaraj.CKb | 2017 | Materials Today: Proceedings 4 (2017) 10994–11003 | optimization | optimization | Experimental Optimization | ||||
| 2017 | O24 | Studies on Parametric Optimization for Fused Deposition Modelling Process | Vijay.B.Nidagundia*, R.Keshavamurthyb,C.P.S.Prakashc | 2017 | Materials Today: Proceedings 2 ( 2015 ) 1691 – 1699 | optimization | optimization | Experimental Optimization | ||||
| 2017 | O25 | A case study on topology optimized design for additive manufacturing | A W Gebisa* and H G Lemu | 2017 | IOP Conference Series: Materials Science and Engineering, Volume 276, conference 1 | optimization | optimization | TOPOLOGICAL OPTIMIZATION | ||||
| 2019 | O26 | Self-supporting structure design in additive manufacturing through explicit topology optimization ( de 97) | Xu Guo and Jianhua Zhou and Weisheng Zhang and Zongliang Du and Chang Liu and Ying Liu | 2017 | Comput. Methods Appl. Mech. Engrg. 323 (2017) 27–63 | optimization | optimization | TOPOLOGICAL OPTIMIZATION | ||||
| 2019 | O27 | Topology optimization of self-supporting support structures for additive manufacturing | Francesco Mezzadri and Vladimir Bouriakov and Xiaoping Qian | 2018 | Additive Manufacturing 21 (2018) 666–682 | optimization | optimization | TOPOLOGICAL OPTIMIZATION | ||||
| 2019 | O28 | Shape optimization of a layer by layer mechanical constraint for additive manufacturing | Gr{\'{e}}goire Allaire and Charles Dapogny and Alexis Faure and Georgios Michailidis | 2017 | C. R.Acad.Sci.Paris,Ser.I355(2017)699–717 | optimization | optimization | TOPOLOGICAL OPTIMIZATION | ||||
| 2019 | O29 | Support structure design in additive manufacturing based on topology optimization | Kuo, Yu-Hsin and Cheng, Chih-Chun and Lin, Yang-Shan and San, Cheng-Hung | 2018 | Struct Multidisc Optim (2018) 57:183–195 | optimization | optimization | TOPOLOGICAL OPTIMIZATION | ||||
| 2019 | O30 | Mechanical response of a triply periodic minimal surface cellular structures manufactured by selective laser melting | Yang, L., Yan, C., Han, C., (...), Yang, S., Shi, Y. | 2018 | International Journal of Mechanical Sciences 148, pp. 149-157 | optimization | optimization | LATTICE, MECHANICS | ||||
| 2019 | O31 | 3D printing assisted finite element analysis for optimising the manufacturing parameters of a lumbar fusion cage | Provaggi, E., Capelli, C., Rahmani, B., Burriesci, G., Kalaskar, D.M. | 2019 | Materials and Design 163,10754 | optimization | optimization | LATTICE, MECHANICS | ||||
| 2019 | O32 | Insights into the mechanical properties of several triply periodic minimal surface lattice structures made by polymer additive manufacturing | Maskery, I., Sturm, L., Aremu, A.O., (...), Ashcroft, I.A., Hague, R.J.M. | 2018 | Polymer 152, pp. 62-71 | optimization | optimization | LATTICE, MECHANICS | ||||
| 2019 | O33 | Topology optimization for functionally graded cellular composites with metamaterials by level sets | Li, H., Luo, Z., Gao, L., Walker, P. | 2018 | Computer Methods in Applied Mechanics and Engineering 328, pp. 340-364 | optimization | optimization | TOPOLOGICAL, LATTICE, MECHANICS | ||||
| 2019 | O34 | Minimum compliance topology optimization of shell–infill composites for additive manufacturing | Wu, J., Clausen, A., Sigmund, O. | 2017 | Computer Methods in Applied Mechanics and Engineering 326, pp. 358-375 | optimization | optimization | TOPOLOGICAL, LATTICE, MECHANICS | ||||
| 2019 | O35 | Topology optimization for multiscale design of porous composites with multi-domain microstructures | Gao, J., Luo, Z., Li, H., Gao, L. | 2019 | Computer Methods in Applied Mechanics and Engineering 344, pp. 451-476 | optimization | optimization | TOPOLOGICAL, LATTICE, MECHANICS | ||||
| 2019 | O36 | Automatic reconstruction of beam structures from 3D topology optimization results | Nana, A., Cuillière, J.-C., Francois, V. | 2017 | Computers and Structures 189, pp. 62-82 | optimization | optimization | Topology, Mechanics (NOT AM) | ||||
| 2019 | O37 | An overview of functionally graded additive manufacturing | Loh, G.H., Pei, E., Harrison, D., Monzón, M.D. | 2018 | Additive Manufacturing 23, pp. 34-44 | optimization | optimization | Optimization of Mechanical Function (Porous) | ||||
| 2019 | O38 | Dynamic multiscale topology optimization for multi-regional micro-structured cellular composites | Gao, J., Luo, Z., Li, H., Li, P., Gao, L. | 2019 | Composite Structures 211, pp. 401-417 | optimization | optimization | TOPOLOGICAL, LATTICE, MECHANICS | ||||
| 2019 | O39 | Exploiting Additive Manufacturing Infill in Topology Optimization for Improved Buckling Load | Clausen, A., Aage, N., Sigmund, O. | 2016 | Engineering 2(2), pp. 250-257 | optimization | optimization | TOPOLOGICAL, MECHANICAL | ||||
| 2019 | O40 | Topology optimization for additive manufacturing using a component of a humanoid robot | Junk, S., Klerch, B., Nasdala, L., Hochberg, U. | 2018 | Procedia CIRP 70, pp. 102-107 | optimization | optimization | TOPOLOGICAL | ||||
| 2019 | O41 | Direct Bio-printing with Heterogeneous Topology Design | Ahsan, A.M.M.N., Xie, R., Khoda, B. | 2017 | Procedia Manufacturing 10, pp. 945-956 | optimization | optimization | Heterogeneous Topology | ||||
| 2019 | O42 | Multi-Objective Optimization of Additive Manufacturing Process | Asadollahi-Yazdi, E., Gardan, J., Lafon, P. | 2018 | IFAC-PapersOnLine 51(11), pp. 152-157 | optimization | Methodology, surface, mechanics, optimization, manufacturing | Mechanical characterization, finish, manufacturability/manufacturing. | ||||
| 2021 | O43 | Integrated topology optimization of multi-component structures considering connecting interface behavior | Pai Liu and Zhan Kang | 2018 | Comput. Methods Appl. Mech. Engrg. 341 (2018) 851–887 | optimization | - | Topological optimization, multimaterial | ||||
| 2021 | O44 | {OAPS}: An Optimization Algorithm for Part Separation in Assembly Design for Additive Manufacturing | Angshuman Deka, Sara Behdad | 2018 | Proceedings Article published 26 Aug 2018 in Volume 4: 23rd Design for Manufacturing and the Life Cycle Conference; 12th International Conference on Micro- and Nanosystems | optimization, Manufacturing process cases (general and specific) | optimization, manufacturing, assembly | ensemble, algorithm, manufacturing time optimization | ||||
| 2021 | O45 | Interactive Topology Optimization | Nobel-Jørgensen, Morten; Bærentzen, Jakob Andreas | 2016 | Technical University of Denmark Department of Applied Mathematics and Computer Science, DTU Compute PHD-2015, No. 375 | optimization | optimization | Topological Optimization | ||||
| 2021 | O45 A | TopOpt DTU | TOPOPT GROUP | 2016, 2021 | https://www.topopt.mek.dtu.dk/ | optimization | optimization | Topological Optimization | ||||
| 2017 | D1 | DOE Based Parametric Study of Volumetric Change of FDM Parts | Pavan Kumar Gurrala*, Srinivasa Prakash Regalla | 2014 | Procedia Materials Science 6 ( 2014 ) 354 – 360 | Dimensional modeling | dimension | deer | ||||
| 2017 | D2 | Design for manufacturing of surfaces to improve accuracy in Fused Deposition Modeling | Alberto Boschetto a,n, LuanaBottini a | 2016 | RoboticsandComputer-IntegratedManufacturing37(2016)103–114 | Dimensional modeling | dimension | CNC code, dimensional, simulation, model correction | ||||
| 2017 | D3 | Analysis of dimensional performance for a 3D open-source printer based on fused deposition modeling technique | L. M. Galantuccia, I. Bodib,*, J. Kacanib, F. Lavecchiaa | 2015 | Procedia CIRP 28 ( 2015 ) 82 – 87 | Dimensional modeling | dimension | deer | ||||
| 2017 | D4 | Dimensional tolerances for additive manufacturing: Experimental investigation for Fused Deposition Modeling | Tobias Lienekea,b, Vera Denzera*, Guido A. O. Adama,b, Detmar Zimmera | 2016 | Procedia CIRP 43 ( 2016 ) 286 – 291 | Dimensional modeling | dimension | deer | ||||
| 2017 | D5 | Fast Deviation Simulation for 'Fused Deposition Modeling' process | Mahmood, Shahraina*, Talamona, Didierac, Goh, Kheng Lima, Qureshi, A.J.b | 2016 | Procedia CIRP 43 ( 2016 ) 327 – 332 | Dimensional modeling | dimension | deer | ||||
| 2017 | D6 | Improving dimensional accuracy of Fused Deposition Modelling processed part using grey Taguchi method | Anoop Kumar Sood a, R.K. Ohdar b, S.S. Mahapatra c,* | 2009 | Materials and Design 30 (2009) 4243–4252 | Dimensional modeling | dimension | deer | ||||
| 2017 | D7 | Benchmarking of FDM machines through part quality using IT grades | Paolo Minetolaa,*, Luca Iulianoa, Giovanni Marchiandia | 2016 | Procedia CIRP 41 ( 2016 ) 1027 – 1032 | Dimensional modeling | dimension | Benchmarking | ||||
| 2017 | D8 | Effect of processing conditions on the bonding quality of FDM polymer filaments | Q. Sun, G.M. Rizvi, C.T. Bellehumeur, P. Gu, | 2008 | Rapid Prototyping Journal, Vol. 14 Issue: 2,pp. 72-80, | Dimensional modeling | dimension | modeling | ||||
| 2017 | D9 | Deviation Modeling and Shape transformation in Design for Additive Manufacturing | Zuowei Zhu and Nabil Anwer and Luc Mathieu | 2017 | Procedia CIRP 60 ( 2017 ) 211 – 216 | Dimensional modeling | dimension | MODELING, simulation of deviations | ||||
| 2019 | D10 | Investigation of part distortions as a result of hybrid manufacturing | Zhu, Z., Dhokia, V., Nassehi, A., Newman, S.T. | 2016 | Robotics and Computer-Integrated Manufacturing 37,1348, pp. 23-32 | Dimensional modeling | dimension | Hybrid processes, modeling, doe | ||||
| 2019 | D11 | In-situ observation and numerical simulation on the transient strain and distortion prediction during additive manufacturing | Xie, R., Chen, G., Zhao, Y., (...), Lin, X., Shi, Q. | 2019 | Journal of Manufacturing Processes 38, pp. 494-501 | Dimensional modeling | dimension | MODELING, simulation of deviations | ||||
| 2019 | D12 | A challenge for enhancing the dimensional accuracy of a low-cost 3D printer by means of self-replicated parts | Minetola, P., Galati, M. | 2018 | Additive Manufacturing 22, pp. 256-264 | Dimensional modeling | dimension | modeling, doe, process control | ||||
| 2019 | D13 | Dimensional and form errors of PC parts printed via Fused Deposition Modelling | Reyes-Rodríguez, A., Dorado-Vicente, R., Mayor-Vicario, R. | 2017 | Procedia Manufacturing 13, pp. 880-887 | Dimensional modeling | dimension | modeling, doe | ||||
| 2019 | D14 | Dimensional accuracy of threads manufactured by fused deposition modeling | Tronvoll, S.A., Elverum, C.W., Welo, T. | 2018 | Procedia Manufacturing 26, pp. 763-773 | Dimensional modeling | dimension | modeling, doe | ||||
| 2021 | D15 | Analysis of the factors affecting the dimensional accuracy of 3D printed products | Kushagra Tiwari and Santosh Kumar | 2018 | Journal Article published 2018 in Materials Today: Proceedings volume 5 issue 9 on pages 18674 to 18680 | Dimensional modeling | dimension | modeling, doe | ||||
| 2021 | D16 | INTERNATIONAL STANDARD ISO 286-1. Geometrical product specifications(GPS) — ISO code system for toleranceson linear sizes —Part 1: Basis of tolerances, deviations and fits | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2010 | https://www.iso.org/obp/ui/#iso:std:iso:286:-1:ed-2:v1:en | Dimensional modeling | dimension | norms | ||||
| 2021 | D17 | ESTUDIO EXPERIMENTAL PARA MEJORAR LA PRECISIÓN DIMENSIONAL Y SUPERFICIAL DE PIEZAS FABRICADAS MEDIANTE MODELADO POR DEPOSICIÓN FUNDIDA | JORGE ANDRÉS MARTÍNEZ MERCADO, DAVID ENRIQUE SEPÚLVEDA FLÓREZ | 2021 | UNIVERSIDAD DEL ATLÁNTICO, FACULTAD DE INGENIERÍA, PROGRAMA DE INGENIERÍA MECÁNICA | Dimensional modeling, surface modeling, manufacturing | dimension, surface, manufacturing | Tolerances, finishes, multimaterial, multiprocess, process chain. | ||||
| 2021 | D18 | Estudio Experimental De Los Procesos De Mecanizado Para Mejorar El Acabado Superficial y Tolerancias De Las Piezas Impresas En 3D | Alberto Enrique Alonso De la Hoz, Cristian Camilo Coronado Santiago | 2021 | UNIVERSIDAD DEL ATLÁNTICO, FACULTAD DE INGENIERÍA, PROGRAMA DE INGENIERÍA MECÁNICA | Dimensional modeling, surface modeling, manufacturing | dimension, surface, manufacturing | Tolerances, finishes, multimaterial, multiprocess, process chain, machining. | ||||
| 2021 | D19 | Accuracy prediction in fused deposition modeling | A. Boschetto & L. Bottini | 2014 | Int J Adv Manuf Technol (2014) 73:913–928 | Dimensional modeling | dimension | tolerances | ||||
| 2021 | D20 | Research on the warping deformation in fused deposition modeling. | Xin Li, Zhuo Wang, Jianzhong Shang. | 2016 | Asian Journal of Research in Chemistry and Pharmaceutical Sciences, 2016, 4(1): 21–30. | Dimensional modeling | dimension | tolerances | ||||
| 2017 | S1 | Representation of surface roughness in fused deposition modeling | Daekeon Ahna,∗, Jin-Hwe Kweona, Soonman Kwonb, Jungil Songb, Seokhee Leec | 2009 | Journal of Materials Processing Technology 209 (2009) 5593–5600 | surface modeling | surface | Characterization, simulation or analytical model of roughness | ||||
| 2017 | S2 | Quantitative analysis of surface profile in fused deposition modelling | Yu-an Jina,c, Hui Lib, Yong Hea,c,∗, Jian-zhong Fua, | 2015 | Additive Manufacturing 8 (2015) 142–148 | surface modeling | surface | Characterization, simulation or analytical model of deviation per unit area | ||||
| 2017 | S3 | Experimental study aiming to enhance the surface finish of fused deposition modeled parts | L.M. Galantucci (1)*, F. Lavecchia, G. Percoco | 2009 | CIRP Annals - Manufacturing Technology 58 (2009) 189–192 | surface modeling, manufacturing | surface, manufacturing | Chemical attack, multiprocess, process chain | ||||
| 2017 | S4 | Dimensional and surface texture characterization in Fused Deposition Modelling (FDM) with ABS plus | P.J. Nuñeza,*, A. Rivasa, E. García-Plazaa, E. Beamudb, A. Sanz-Loberac | 2015 | Procedia Engineering 132 ( 2015 ) 856 – 863 | surface modeling | surface | finished and dimensional precision | ||||
| 2017 | S5 | Roughness prediction in coupled operations of fused depositionmodeling and barrel finishing | Alberto Boschetto∗, Luana Bottini | 2015 | Journal of Materials Processing Technology 219 (2015) 181–192 | surface modeling, manufacturing | surface, manufacturing | Experimental characterization and analytical simulation of drilling, process chain, multiprocess. | ||||
| 2017 | S6 | Finishing of Fused Deposition Modeling parts by CNC machining | Alberto Boschetto,LuanaBottini n, FrancescoVeniali | 2016 | Robotics and Computer-Integrated Manufacturing 41(2016)92–101 | surface modeling, manufacturing | surface, manufacturing | Experimental characterization and analytical simulation of CNC milling, process chain, multiprocess. | ||||
| 2017 | S7 | Improvement of surface finish by staircase machining in fused deposition modeling | Pulak M. Pandey, N. Venkata Reddy, Sanjay G. Dhande | 2003 | Journal of Materials Processing Technology 132(1-3), pp. 323-331 | surface modeling, manufacturing | surface, manufacturing | hot modeling and machining, process chain, multiprocess | ||||
| 2017 | S8 | Integration of FDM surface quality modeling with process | Alberto Boschetto, Luana Bottini∗, Francesco Veniali | 2016 | Additive Manufacturing 12, pp. 334-344 | surface modeling | surface | Characterization and modeling | ||||
| 2017 | S9 | Surface improvement of fused deposition modeling parts by barrel finishing | Alberto Boschetto, Luana Bottini | 2015 | Rapid Prototyping Journal, Vol. 21 Issue: 6,pp. 686-696, | surface modeling, manufacturing | surface, manufacturing | Modeling and drilling, process chain, multiprocess | ||||
| 2017 | S10 | Investigations for improving the surface finish of FDM based ABS replicas by chemical vapor smoothing process: a case study | Jaspreet Singh, Rupinder Singh, Harwinder Singh | 2017 | Assembly Automation, Vol. 37 Issue: 1,pp. 13-21 | surface modeling, manufacturing | surface, manufacturing | Modeling and steam bath, process chain, multiprocess. | ||||
| 2017 | S11 | Pre and post processing techniques to improve surface characteristics of FDM parts: a state of art review and future applications | Jasgurpreet Singh Chohan, Rupinder Singh, | 2017 | Rapid Prototyping Journal 23(3), pp. 495-513 | surface modeling, manufacturing | surface, manufacturing | State of the art (pre and post processed FDM surface), process chain, multiprocess. | ||||
| 2017 | S12 | Surface texture metrology for metal additive manufacturing: a review | Townsend, A., Senin, N., Blunt, L., Leach, R.K., Taylor, J.S. | 2016 | Precision Engineering 46, pp. 34-47 | surface modeling | surface | State of the art (finished in metal) | ||||
| 2017 | S13 | Machining of Additively Manufactured Parts: Implications for Surface Integrity | Olusola Oyelola and Peter Crawforth and Rachid M{\textquotesingle}Saoubi and Adam T. Clare | 2016 | Procedia CIRP 45 ( 2016 ) 119 – 122 | surface modeling | surface | modeling and machining | ||||
| 2019 | S14 | Modeling of the chemical finishing process for polylactic acid parts in fused deposition modeling and investigation of its tensile properties | Jin, Y., Wan, Y., Zhang, B., Liu, Z. | 2017 | Journal of Materials Processing Technology 240, pp. 233-239 | surface modeling, manufacturing | surface, manufacturing | Chemical attack, multiprocess, process chain | ||||
| 2019 | S15 | Optimizing Surface texture and coating thickness of nickel coated ABS-3D parts | Khan, M.S., Mishra, S.B., Kumar, M.A., Banerjee, D. | 2018 | Materials Today: Proceedings 5(9), pp. 19011-19018 | surface modeling, manufacturing | surface, manufacturing | Coated, process chain | ||||
| 2021 | S16 | Analysis of the influence of chemical treatment to the strength and surface roughness of {FDM} | R. H. Hambali and K. M. Cheong and N. Azizan | 2017 | Journal Article published Jun 2017 in IOP Conference Series: Materials Science and Engineering volume 210 on page 012063 | Mechanical modeling, surface modeling, Cases of manufacturing processes (general and specific) | mechanics, surface, manufacturing | Mechanical characterization, chemical attack, process chain. | ||||
| 2021 | S17 | Hybrid estimation of surface roughness distribution in FDMparts using analytical modeling and empirical investigation | Vahabli, E. and Rahmati, S. | 2017 | The International Journal of Advanced Manufacturing Technology, Vol. 88 Nos 5/8, pp. 2287-2303. | surface modeling | surface | Surface modeling | ||||
| 2021 | S18 | Modelling micro geometrical profiles in fused deposition process | A. Boschetto & V. Giordano & F. Veniali | 2012 | Int J Adv Manuf Technol (2012) 61:945–956 | surface modeling | surface | Surface modeling | ||||
| 2021 | S19 | Surface roughness prediction in fused deposition modelling by neural networks | A. Boschetto & V. Giordano & F. Veniali | 2013 | Int J Adv Manuf Technol (2013) 67:2727–2742 | surface modeling | surface | Surface modeling | ||||
| 2017 | F1 | A new part consolidation method to embrace the design freedom of additive manufacturing | Sheng Yang, Yunlong Tang, Yaoyao Fiona Zhao∗ | 2015 | Journal of Manufacturing Processes 20 (2015) 444–449 | Cases of manufacturing processes (general and specific) | Manufacturing | DFAM | ||||
| 2017 | F2 | Design for Additive Manufacturing – Supporting the Substitution of Components in Series Products | Christoph Klahn*, Bastian Leutenecker, Mirko Meboldt | 2014 | Procedia CIRP 21 ( 2014 ) 138 – 143 | Cases of manufacturing processes (general and specific) | Manufacturing | DFAM | ||||
| 2017 | F3 | Design Strategies for the Process of Additive Manufacturing | Christoph Klahna*, Bastian Leuteneckerb, Mirko Meboldtb | 2015 | Procedia CIRP 36 ( 2015 ) 230 – 235 | Cases of manufacturing processes (general and specific) | Manufacturing | DFAM | ||||
| 2017 | F4 | Fluid-based removal of inner support structures manufactured by fused deposition modeling: an investigation on factors of influence | Mario Lusic,*, Frank Feuersteina, Driton Morinaa, Rüdiger Hornfecka | 2016 | Procedia CIRP 41 ( 2016 ) 1033 – 1038 | Cases of manufacturing processes (general and specific) | Manufacturing | support removal | ||||
| 2017 | F5 | Component Replication using 3D Printing Technology | Dr. B.Satyanarayanaa*, Kode Jaya Prakashb | 2015 | Procedia Materials Science 10 ( 2015 ) 263 – 269 | Cases of manufacturing processes (general and specific) | Manufacturing | reverse engineering | ||||
| 2017 | F6 | Manufacturing of PMMA Cam Shaft by Rapid Prototyping | Jaiganesh .V*, Andrew anthony christopher 1, Mugilan E2 | 2014 | Procedia Engineering 97 ( 2014 ) 2127 – 2135 | Cases of manufacturing processes (general and specific) | Manufacturing | Case study: crankshaft | ||||
| 2017 | F7 | 3D printed wind turbines part 1: Design considerations and rapid manufacture potential | K. Bassett ⇑, R. Carriveau, D.S.-K. Ting | 2015 | Sustainable Energy Technologies and Assessments 11 (2015) 186–193 | Cases of manufacturing processes (general and specific) | Manufacturing | Case study: wind turbine | ||||
| 2017 | F8 | 3D Printing, a Maturing Technology | Karel Brans | 2013 | 11th IFAC Workshop on Intelligent Manufacturing Systems The International Federation of Automatic Control May 22-24, 2013. São Paulo, Brazil | Cases of manufacturing processes (general and specific) | Manufacturing | Advantages, disadvantages and information management software. | ||||
| 2017 | F9 | A critical review of the use of 3-D printing in the construction industry | Peng Wua,⁎,1, JunWangb, XiangyuWangb | 2016 | Automation in Construction 68 (2016) 21–31 | Cases of manufacturing processes (general and specific) | Manufacturing | The state of the art construction. | ||||
| 2017 | F10 | Development of a mobile fused deposition modeling system with enhanced manufacturing flexibility | Jae-Won Choia,b, Francisco Medinaa,b, Chiyen Kima, David Espalina,b, David Rodrigueza,b, Brent Stuckerc, Ryan Wickera,b,∗ | 2011 | Journal of Materials Processing Technology 211 (2011) 424–432 | Cases of manufacturing processes (general and specific) | Manufacturing | Mobile printing system (remote) | ||||
| 2017 | F11 | The potential to enhance membrane module design with 3D printing technology | Jian-YuanLee a,b,c,1, WenSeeTan a,b,c,1, JiaAn c, CheeKaiChua c, ChuyangY.Tang d, AnthonyG.Fane b,e, TzyyHaurChong b,e,n | 2016 | Journal ofMembraneScience499(2016)480–490 | Cases of manufacturing processes (general and specific) | Manufacturing | membrane | ||||
| 2017 | F12 | Large-scale 3D printing of ultra-high performance concrete – a new processing route for architects and builders | C. Gosselin a,b, R. Duballet a,b, Ph. Roux a,b, N. Gaudillière a,b, J. Dirrenberger a,c,⁎, Ph. Morel a,d,b | 2016 | Materials and Design 100 (2016) 102–109 | Cases of manufacturing processes (general and specific) | Manufacturing | construction (concrete) | ||||
| 2017 | F13 | MASK-DIRECTED MICRO-3D PRINTING | Derek S. Hernandez, Jason B. Shear | 2014 | capitulo de libro | Cases of manufacturing processes (general and specific) | Manufacturing | microprinting | ||||
| 2017 | F14 | Modelling curved-layered printing paths for fabricating large-scaleconstruction components | Sungwoo Lima,∗, Richard A. Buswellb, Philip J. Valentinec, Daniel Pikerd,Simon A. Austinb, Xavier De Kestelierd | 2016 | Additive Manufacturing 12, pp. 216-230 | Cases of manufacturing processes (general and specific) | Manufacturing | construction | ||||
| 2017 | F15 | Investigation of the effect of built orientation on mechanical properties and total cost of FDM parts | Sandeep Rauta,VijayKumar S. Jattib,*, Nitin K. Khedkarc,T.P.Singhd | 2014 | Procedia Materials Science 6 ( 2014 ) 1625 – 1630 | Cases of manufacturing processes (general and specific) | Manufacturing | orientation and cost | ||||
| 2017 | F16 | An improved fused deposition modeling process for forminglarge-size thin-walled parts | Du Jun∗, Wei Zhengying, Wang Xin, Wang Jijie, Chen Zhen | 2016 | Journal of Materials Processing Technology 234 (2016) 332–341 | Cases of manufacturing processes (general and specific) | Manufacturing | Thin wall | ||||
| 2017 | F17 | INTRODUCTION OF A DESIGN FOR RAPID MANUFACTURING (DFRM) PERSPECTIVE IN ENGINEERING DESIGN EDUCATION | Sandor Campos, Javier Munguía and Joaquim Lloveras | 2007 | INTERNATIONAL CONFERENCE ON ENGINEERING AND PRODUCT DESIGN EDUCATION 13-14 SEPTEMBER 2007, NORTHUMBRIA UNIVERSITY, NEWCASTLE UPON TYNE, UNITED KINGDOM | Cases of manufacturing processes (general and specific) | Manufacturing | engineering design study | ||||
| 2017 | F18 | Pursuing successful rapid manufacturing: a users' best-practices approach | Javier Munguía Joaquim de Ciurana Carles Riba | 2008 | Rapid Prototyping Journal, Vol. 14 Iss 3 pp. 173 - 179 | Cases of manufacturing processes (general and specific) | Manufacturing | design rules | ||||
| 2017 | F19 | Requirements for the Design of flexible and changeable Manufacturing and Assembly Systems: a SME-survey | Pasquale Russo Spenaa, Philipp Holznera*, Erwin Raucha, Renato Vidonia, Dominik T. Matt | 2016 | Procedia CIRP 41 ( 2016 ) 207 – 212 | Cases of manufacturing processes (general and specific) | Manufacturing | manufacturing system design | ||||
| 2017 | F20 | Designing a Modular Rapid Manufacturing Process | Jacquelyn K. S. Nagel, Frank W. Liou | 2010 | Journal of Manufacturing Science and Engineering, DECEMBER 2010, Vol. 132 | Cases of manufacturing processes (general and specific) | Manufacturing | manufacturing system design/design rules | ||||
| 2017 | F21 | Fused deposition modelling based rapid patterns for investment casting applications: a review | Sunpreet Singh, Rupinder Singh | 2016 | Rapid Prototyping Journal 22(1), pp. 123-143 | Cases of manufacturing processes (general and specific) | Manufacturing | State of the art (casting)/design rule | ||||
| 2017 | F22 | Investigations for statistically controlled investment casting solution of FDM-based ABS replicas | Rupinder Singh, Gurwinder Singh | 2014 | Rapid Prototyping Journal, Vol. 20 Issue: 3,pp. 215-220 | Cases of manufacturing processes (general and specific) | Manufacturing | casting/design rule | ||||
| 2017 | F23 | Development of rapid tooling using fused deposition modeling: a review | Kamaljit Singh Boparai, Rupinder Singh, Harwinder Singh, | 2016 | Rapid Prototyping Journal, Vol. 22 Issue: 2,pp. 281-299 | Cases of manufacturing processes (general and specific) | Manufacturing | State of the art (RT)/design rule | ||||
| 2017 | F24 | Study of the complementary usages of selective laser sintering during the high volume production of plastic parts | Tomaz Brajlih, Matej Paulic, Tomaz Irgolic, Ziga Kadivnik, Joze Balic, Igor Drstvensek | 2016 | Rapid Prototyping Journal 22(4), pp. 735-742 | Cases of manufacturing processes (general and specific) | Manufacturing | high production/design rule | ||||
| 2017 | F25 | Options for additive rapid prototyping methods (3D printing) in MEMS technology | Victor A. Lifton, Gregory Lifton, Steve Simon, | 2014 | Rapid Prototyping Journal, Vol. 20 Issue: 5,pp. 403-412 | Cases of manufacturing processes (general and specific) | Manufacturing | State of the art (MEMS)/design rule | ||||
| 2017 | F26 | A review of melt extrusion additive manufacturing processes: I. Process design and modeling | Turner, B.N., Strong, R., Gold, S.A. | 2014 | Rapid Prototyping Journal 20(3),17111231, pp. 192-204 | Cases of manufacturing processes (general and specific) | Manufacturing | State of the art (melt AM)/design rule | ||||
| 2017 | F27 | A review of melt extrusion additive manufacturing processes: II. Materials, dimensional accuracy, and surface roughness | Brian N. Turner, Scott A Gold, | 2015 | Rapid Prototyping Journal, Vol. 21 Issue: 3,pp. 250-261 | Cases of manufacturing processes (general and specific) | Manufacturing | State of the art (melt AM)/design rule | ||||
| 2017 | F28 | Some investigations for small-sized product fabrication with FDM for plastic components | Rupinder Singh | 2013 | Rapid Prototyping Journal, Vol. 19 Issue: 1,pp. 58-63, | Cases of manufacturing processes (general and specific) | Manufacturing | small batch production | ||||
| 2017 | F29 | Implementation of rapid manufacturing for mass customisation | Dominik Deradjat, Tim Minshall | 2017 | Journal of Manufacturing Technology Management, Vol. 28 Issue: 1,pp. 95-121 | Cases of manufacturing processes (general and specific) | Manufacturing | State of the art (Customization/mass production) | ||||
| 2017 | F30 | Integrating stereolithography and direct print technologies for 3D structural electronics fabrication | Amit Joe Lopes, Eric MacDonald, Ryan B. Wicker, | 2012 | Rapid Prototyping Journal, Vol. 18 Issue: 2,pp. 129-143 | Cases of manufacturing processes (general and specific) | Manufacturing | MEMS | ||||
| 2017 | F31 | Flow-based fabrication: An integrated computational workflow for design and digital additive manufacturing of multifunctional heterogeneously structured objects | Duro-Royo, J., Mogas-Soldevila, L., Oxman, N. | 2015 | CAD Computer Aided Design 69, pp. 143-154 | Cases of manufacturing processes (general and specific) | Manufacturing | Design and manufacture of multifunctional structure | ||||
| 2017 | F32 | Web-based rapid prototyping and manufacturing systems: A review | Lan, H. | 2009 | Computers in Industry 60(9), pp. 643-656 | Cases of production and manufacturing processes (general and specific) | Manufacturing | State of the art (web-based manufacturing systems) | ||||
| 2017 | F33 | Composites Part Production with Additive Manufacturing Technologies | Daniel-Alexander Türk and Ralph Kussmaul and Markus Zogg and Christoph Klahn and Bastian Leutenecker-Twelsiek and Mirko Meboldt | 2017 | Procedia CIRP 66 ( 2017 ) 306 – 311 | Cases of production and manufacturing processes (general and specific) | Manufacturing | Manufacturing of composite with AM | ||||
| 2019 | F34 | Enhancement of surface reflectivity of fused deposition modeling parts by post-processing | Chen, Y.-F., Wang, Y.-H., Tsai, J.-C. | 2019 | Optics Communications 430, pp. 479-485 | Cases of production and manufacturing processes (general and specific) | Manufacturing | Optics, multiprocessing | ||||
| 2019 | F35 | Additive manufacturing of biomaterials | Bose, S., Ke, D., Sahasrabudhe, H., Bandyopadhyay, A. | 2018 | Progress in Materials Science 93, pp. 45-111 | Cases of production and manufacturing processes (general and specific) | Manufacturing | Biomaterials, multimaterials | ||||
| 2019 | F36 | 3D-printed steel reinforcement for digital concrete construction – Manufacture, mechanical properties and bond behaviour | Mechtcherine, V., Grafe, J., Nerella, V.N., (...), Hertel, M., Füssel, U. | 2018 | Construction and Building Materials 179, pp. 125-137 | Cases of production and manufacturing processes (general and specific) | Manufacturing | construction, multimaterial | ||||
| 2019 | F37 | Hybrid additive manufacturing technologies - An analysis regarding potentials and applications | Merklein, M., Junker, D., Schaub, A., Neubauer, F. | 2016 | Physics Procedia 83, pp. 549-559 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, hybrid | ||||
| 2019 | F38 | Developments in construction-scale additive manufacturing processes | Lim, S., Buswell, R.A., Le, T.T., (...), Gibb, A.G.F., Thorpe, T. | 2012 | Automation in Construction 21(1), pp. 262-268 | Cases of production and manufacturing processes (general and specific) | Manufacturing | construction | ||||
| 2019 | F39 | Classification of building systems for concrete 3D printing | Duballet, R., Baverel, O., Dirrenberger, J. | 2017 | Automation in Construction 83, pp. 247-258 | Cases of production and manufacturing processes (general and specific) | Manufacturing | construction | ||||
| 2019 | F40 | Applications of additive manufacturing in the construction industry – A forward-looking review | Delgado Camacho, D., Clayton, P., O'Brien, W.J., (...), Ferron, R., Salamone, S. | 2018 | Automation in Construction 89, pp. 110-119 | Cases of production and manufacturing processes (general and specific) | Manufacturing | construction | ||||
| 2019 | F41 | The utilization of selective laser melting technology on heat transfer devices for thermal energy conversion applications: A review | Jafari, D., Wits, W.W. | 2018 | Renewable and Sustainable Energy Reviews 91, pp. 420-442 | Cases of production and manufacturing processes (general and specific) | Manufacturing | Termofluids, electricity generation | ||||
| 2019 | F42 | 3D printing for rapid sand casting—A review | Upadhyay, M., Sivarupan, T., El Mansori, M. | 2017 | Journal of Manufacturing Processes 29, pp. 211-220 | Cases of production and manufacturing processes (general and specific) | Manufacturing | casting | ||||
| 2019 | F43 | Development and surface improvement of FDM pattern based investment casting of biomedical implants: A state of art review | Singh, D., Singh, R., Boparai, K.S. | 2018 | Journal of Manufacturing Processes 31, pp. 80-95 | Cases of production and manufacturing processes (general and specific) | Manufacturing | medical, casting | ||||
| 2019 | F44 | A novel 6-axis hybrid additive-subtractive manufacturing process: Design and case studies | Li, L., Haghighi, A., Yang, Y. | 2018 | Journal of Manufacturing Processes 33, pp. 150-160 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, hybrid | ||||
| 2019 | F45 ( 327) | Criteria selection for a comparative study of functional performance of Fused Deposition Modelling and Vacuum Casting processes | Valerga Puerta, A.P., Sanchez, D.M., Batista, M., Salguero, J. | 2018 | Journal of Manufacturing Processes 35, pp. 721-727 | Cases of production and manufacturing processes (general and specific) | Manufacturing | Multi-criteria function, process comparison. | ||||
| 2019 | F46 | Correlations between Influencing Parameters and Quality Properties of Components Produced by Fused Deposition Modeling | Bähr, F., Westkämper, E. | 2018 | Procedia CIRP 72, pp. 1214-1219 | Cases of production and manufacturing processes (general and specific) | Manufacturing, design process, dimension | Design rule, tolerance | ||||
| 2019 | F47 | Design, Development and Experimental Investigation of E-jet Based Additive Manufacturing Process | Kumar Ball, A., Das, R., Das, D., Shekhar Roy, S., Murmu, N.C. | 2018 | Materials Today: Proceedings 5(2), pp. 7355-7362 | Cases of production and manufacturing processes (general and specific) | Manufacturing | Design rule, limitations, advantages | ||||
| 2019 | F48 | Additive Manufacturing Techniques in Manufacturing -An Overview | Prakash, K.S., Nancharaih, T., Rao, V.V.S. | 2018 | Materials Today: Proceedings 5(2), pp. 3873-3882 | Cases of production and manufacturing processes (general and specific) | Manufacturing | technological review | ||||
| 2019 | F49 | A Review on Transition in the Manufacturing of Mechanical Components from Conventional Techniques to Rapid Casting Using Rapid Prototyping | Thomas, P.A., Aahlada, P.K., Kiran, N.S., Ivvala, J. | 2018 | Materials Today: Proceedings 5(5), pp. 11990-12002 | Cases of production and manufacturing processes (general and specific) | Manufacturing | Comparison processes, casting | ||||
| 2019 | F50 | Hybrid manufacturing – integrating traditional manufacturers with additive manufacturing (AM) supply chain | Strong, D., Kay, M., Conner, B., Wakefield, T., Manogharan, G. | 2018 | Additive Manufacturing 21, pp. 159-173 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, hybrid | ||||
| 2019 | F51 | Invited review article: Strategies and processes for high quality wire arc additive manufacturing | Cunningham, C.R., Flynn, J.M., Shokrani, A., Dhokia, V., Newman, S.T. | 2018 | Additive Manufacturing 22, pp. 672-686 | Cases of production and manufacturing processes (general and specific) | Manufacturing | Design rules, process design, limitations | ||||
| 2019 | F52 | 3D printing trends in building and construction industry: a review | Tay, Y.W.D., Panda, B., Paul, S.C., (...), Tan, M.J., Leong, K.F. | 2017 | Virtual and Physical Prototyping 12(3), pp. 261-276 | Cases of production and manufacturing processes (general and specific) | Manufacturing | construction | ||||
| 2019 | F53 | Assessment of mechanical properties of Ni-coated ABS plastics using FDM process | Kannan, S., Senthilkumaran, D. | 2014 | International Journal of Mechanical and Mechatronics Engineering 14(3), pp. 30-35 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, hybrid | ||||
| 2019 | F54 | Additive construction: State-of-the-art, challenges and opportunities | Labonnote, N., Rønnquist, A., Manum, B., Rüther, P. | 2016 | Automation in Construction 72, pp. 347-366 | Cases of production and manufacturing processes (general and specific) | Manufacturing | construction | ||||
| 2019 | F55 | The development of a rapid prototyping prosthetic socket coated with a resin layer for transtibial amputees | Hsu, L.H., Huang, G.F., Lu, C.T., Hong, D.Y., Liu, S.H. | 2010 | Prosthetics and Orthotics International 34(1), pp. 37-45 | Cases of production and manufacturing processes (general and specific) | Manufacturing, mechanics, medical | multiprocess, hybrid, medical | ||||
| 2019 | F56 | A review: additive manufacturing for active electronic components | Saengchairat, N., Tran, T., Chua, C.-K. | 2017 | Virtual and Physical Prototyping 12(1), pp. 31-46 | Cases of production and manufacturing processes (general and specific) | Manufacturing | electronica | ||||
| 2019 | F57 | A review of printed passive electronic components through fully additive manufacturing methods | Tan, H.W., Tran, T., Chua, C.K. | 2016 | Virtual and Physical Prototyping 11(4), pp. 271-288 | Cases of production and manufacturing processes (general and specific) | Manufacturing | electronica | ||||
| 2019 | F58 | Additive manufacturing of concrete in construction: potentials and challenges of 3D concrete printing | Bos, F., Wolfs, R., Ahmed, Z., Salet, T. | 2016 | Virtual and Physical Prototyping 11(3), pp. 209-225 | Cases of production and manufacturing processes (general and specific) | Manufacturing | construction | ||||
| 2019 | F59 | Analysis of sealing methods for FDM-fabricated parts | Mireles, J., Adame, A., Espalin, D., (...), Zinniel, B., Wicker, R. | 2011 | 22nd Annual International Solid Freeform Fabrication Symposium - An Additive Manufacturing Conference, SFF 2011 pp. 185-196 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, hybrid, sealant | ||||
| 2019 | F60 | Metallization on {FDM} Processed Parts Using Electroless Procedure | Azhar Equbal and Asif Equbal and A.K. Sood | 2014 | Procedia Materials Science | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocessing, hybrid, METALLIZATION | ||||
| 2019 | F61 | A study of post-processing methods for improving the tightness of a part fabricated by fused deposition modeling | Jo, K.-H., Jeong, Y.-S., Lee, J.-H., Lee, S.-H. | 2016 | International Journal of Precision Engineering and Manufacturing 17(11), pp. 1541-1546 | Cases of production and manufacturing processes (general and specific) | Manufacturing, mechanics | multiprocess, hybrid, mechanics | ||||
| 2019 | F62 | Review of reverse engineering systems–current state of the art | Geng, Z., Bidanda, B. | 2017 | Virtual and Physical Prototyping 12(2), pp. 161-172 | Cases of production and manufacturing processes (general and specific) | methodology | Reverse engineering, 3D scanning | ||||
| 2019 | F63 | Fused deposition modeling five-axis additive manufacturing: machine design, fundamental printing methods and critical process characteristics | Shen, H., Diao, H., Yue, S., Fu, J. | 2018 | Rapid Prototyping Journal 24(3), pp. 548-561 | Cases of production and manufacturing processes (general and specific) | Manufacturing, surface | Process control, surface, 5 axes | ||||
| 2019 | F64 | Investigation of influence of heat treatment on mechanical strength of {FDM} printed 3D objects | Wonjin Jo and O-Chang Kwon and Myoung-Woon Moon | 2018 | Rapid Prototyping Journal | Cases of production and manufacturing processes (general and specific) | Manufacturing, mechanics | multiprocess, hybrid, mechanics | ||||
| 2019 | F65 | REVIEW OF ADDITIVE MANUFACTURING TECHNOLOGIES AND CHARACTERIZATION OF ADDITIVE MANUFACTURING MACHINES | SOLOMON EZEIRUAKU | 2015 | Requirements for the Degree of Master of Engineering, Manufacturing Engineering, The University of New Mexico Albuquerque, New Mexico | Cases of production and manufacturing processes (general and specific) | Manufacturing | technological review | ||||
| 2019 | F66 | Ultrasonic additive manufacturing A hybrid production process for novel functional products | Friel, R.J., Harris, R.A. | 2013 | Procedia CIRP 6, pp. 35-40 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, hybrid, mechanics | ||||
| 2021 | F67 | Additive manufacturing of multi-material structures | Amit Bandyopadhyay and Bryan Heer | 2018 | Journal Article published Jul 2018 in Materials Science and Engineering: R: Reports volume 129 on pages 1 to 16 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multimaterial | ||||
| 2021 | F68 | Progress in additive manufacturing on new materials: A review | Li, N., Huang, S., Zhang, G., (...), Shi, G., Blackburn, J. | 2019 | Journal of Materials Science and Technology 35(2), pp. 242-269 | Cases of production and manufacturing processes (general and specific) | Manufacturing | New materials | ||||
| 2021 | F69 | Two-Way 4D Printing: A Review on the Reversibility of 3D-Printed Shape Memory Materials | Amelia Yilin Lee and Jia An and Chee Kai Chua | 2017 | Journal Article published Oct 2017 in Engineering volume 3 issue 5 on pages 663 to 674 | Cases of production and manufacturing processes (general and specific) | Manufacturing | smart materials, review | ||||
| 2021 | F70 | Additive manufacturing (3D printing): A review of materials, methods, applications and challenges | Tuan D. Ngo and Alireza Kashani and Gabriele Imbalzano and Kate T.Q. Nguyen and David Hui | 2018 | Journal Article published Jun 2018 in Composites Part B: Engineering volume 143 on pages 172 to 196 | Cases of production and manufacturing processes (general and specific) | Manufacturing | review of materials, methods and applications | ||||
| 2021 | F71 | Printing with mechanically interlocked extrudates using a custom bi-extruder for fused deposition modelling | Mohammad Abu Hasan Khondoker, Asad Asad, Dan Sameoto | 2017 | Journal Article published 13 Aug 2018 in Rapid Prototyping Journal volume 24 issue 6 on pages 921 to 934 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multimaterial, double extruder | ||||
| 2021 | F72 | Hybrid Processes in Additive Manufacturing | Michael P. Sealy and Gurucharan Madireddy and Robert E. Williams and Prahalada Rao and Maziar Toursangsaraki | 2018 | Journal of Manufacturing Science and Engineering JUNE 2018, Vol. 140 | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocessing, review | ||||
| 2021 | F73 | FDM BEST PRACTICE: Assemblies | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific), dimensional modeling. | Manufacturing, dimension | ensemble, modeling, design rules | ||||
| 2021 | F74 | APPLICATION GUIDE Finishing Touch™ Smoothing Station: Expanding Possibilities | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific), surface modeling. | Manufacturing, surface | Finished surface, process chain, multiprocess, design rules | ||||
| 2021 | F75 | TECHNICAL APPLICATION GUIDE FDM Tooling for Sheet Metal Forming: Hydroforming and Rubber Pad Press | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | Multiprocess, process chain, tool, design rules | ||||
| 2021 | F76 | APPLICATION GUIDE: Injection Blow Molding with FDM | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | Multiprocess, process chain, tool, design rules | ||||
| 2021 | F77 | TECHNICAL APPLICATION GUIDE: Investment Casting with FDM Patterns | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules, reverse casting | ||||
| 2021 | F78 | TECHNICAL APPLICATION GUIDE: FDM For Jigs And Fixtures | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules | ||||
| 2021 | F79 | TECHNICAL APPLICATION GUIDE: Guidelines for Preparing and Painting FDM Parts | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific), surface modeling. | Manufacturing, surface | multiprocess, process chain, tool, PATTERN, design rules, reverse casting, surface finish | ||||
| 2021 | F80 | TECHNICAL APPLICATION GUIDE: FDM FOR SAND CASTING | STRATASYS | 2013 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules, sand mold casting | ||||
| 2021 | F81 | TECHNICAL APPLICATION GUIDE: FDM Patterns for RTV (Rubber) Mold Making | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules, silicone mold | ||||
| 2021 | F82 | TECHNICAL APPLICATION GUIDE: Comparison of Sealing Methods for FDM Materials | STRATASYS | 2014 | STRATASYS | Cases of production and manufacturing processes (general and specific), surface modeling. | Manufacturing, surface | multiprocess, process chain, tool, PATTERN, design rules, reverse casting, surface finish, sealing, fluids | ||||
| 2021 | F83 | TECHNICAL APPLICATION GUIDE: Paper Pulp Molding with FDM Tooling | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | Multiprocess, process chain, tool, PATTERN, design rules, CARDBOARD mold. | ||||
| 2021 | F84 | APPLICATION GUIDE: Thermoforming | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, design rules, thermoforming | ||||
| 2021 | F85 | APPLICATION GUIDE: Manufacturing Tools: Modular Fixtures | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules | ||||
| 2021 | F86 | APPLICATION GUIDE: RTV Molding with Soluble Cores | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules, silicone mold | ||||
| 2021 | F87 | APPLICATION BRIEF: RTV Molding with FDM Patterns | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules, silicone mold | ||||
| 2021 | F88 | TECHNICAL APPLICATION GUIDE: Silicone Molding With FDM Patterns | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules, silicone mold | ||||
| 2021 | F89 | TECHNICAL APPLICATION GUIDE: FDM Sacrificial Cores And Mandrels For Composite Layups | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules, silicone mold | ||||
| 2021 | F90 | APPLICATION GUIDE: Spin Casting | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | multiprocess, process chain, tool, PATTERN, design rules, rotating mold | ||||
| 2021 | F91 | TECHNICAL APPLICATION GUIDE: Surrogate Parts for Design, Manufacturing, Training and Support | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | Spare parts, pattern for adjustments, prototypes, training models. | ||||
| 2021 | F92 | APPLICATION GUIDE: Wind Tunnel Testing | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific) | Manufacturing | models for wind tunnels, fluids | ||||
| 2021 | F93 | Multi-material, multi-technology {FDM}: exploring build process variations | David Espalin and Jorge Alberto Ramirez and Francisco Medina and Ryan Wicker | 2014 | Journal Article published 14 Apr 2014 in Rapid Prototyping Journal volume 20 issue 3 on pages 236 to 244 | Cases of production and manufacturing processes (general and specific), mechanical modeling, surface modeling. | Manufacturing, mechanics, surface | Surface finish, mechanical characterization, printing time, process chain, multiprocess, design rules. | ||||
| 2021 | F94 | SISTEMA DE CODIFICACIÓN DE PIEZAS PARA LA PLANEACIÓN DE PROCESOS METAL MECÁNICOS TRADICIONALES | JOSÉ SALVADOR RUIZ BACA | 2005 | INSTITUTO TECNOLÓGICO Y DE ESTUDIOS SUPERIORES DE MONTERREY CAMPUS ESTADO DE MÉXICO | Cases of production and manufacturing processes (general and specific) | Manufacturing | encoding systems process planning, process chain, multiprocessing | ||||
| 2021 | F95 | Sistemas de Manufactura: Grupos Tecnológicos | Instituto Tecnologico de Chihuahua II | - | Instituto Tecnologico de Chihuahua II | Cases of production and manufacturing processes (general and specific) | Manufacturing | coding systems process planning, process chain, multiprocessing | ||||
| 2021 | F96 | A novel decision-making logic for hybrid manufacture of prismatic components based on existing parts | Zicheng Zhu and Vimal Dhokia and Stephen T. Newman | 2017 | J Intell Manuf (2017) 28:131–148 | Cases of production and manufacturing processes (general and specific), surface modeling, dimensional modeling. | Manufacturing, dimension, surface | multiprocessing, review, finish, tolerances | ||||
| 2021 | F97 | TECHNICAL APPLICATION GUIDE: Comparison of Bonding Methods for FDM Materials | STRATASYS | 2015 | STRATASYS | Cases of production and manufacturing processes (general and specific), mechanical modeling. | Manufacturing, mechanics | multiprocess, process chain, glue, mechanical resistance, mechanical characterization | ||||
| 2021 | F98 | Adhesives technology handbook | Ebnesajjad, Sina and Landrock, Arthur H | 2014 | William Andrew | Cases of production and manufacturing processes (general and specific), mechanical modeling. | Manufacturing, mechanics | multiprocess, process chain, glue, mechanical resistance, mechanical characterization, STATE OF THE ART | ||||
| 2023 | F99 | Part segregation based on particle swarm optimisation for assembly design in additive manufacturing | Maiyar, L.M., Singh, S., Prabhu, V., Tiwari, M.K. | 2019 | International Journal of Computer Integrated Manufacturing. | - | - | - | ||||
| F100 | Part separation technique for assembly-based design in additive manufacturing using genetic algorithm | Deka, A., Behdad, S. | 2019 | Procedia Manufacturing, 34, pp. 764-771 | - | - | - | |||||
| 2017 | ME1 | Aplicaciones de las impresoras 3D en medicina | Jorge Luis Arráez Álvarez. Mª Elena Arráez Álvarez | 2014 | Reduca (Recursos Educativos). Serie Congresos Alumnos. 6 (1): 317-322, 2014 ISSN: 1989-5003 | Medical applications | medicine | tissue printing, bone and drug | ||||
| 2017 | ME2 | Impresoras 3D y la medicina | Cuéllar Rojas, Armando1 | - | 1 MINSAP Nivel Central/Dirección de informática y Comunicaciones, La Habana, Cuba, mandycr@infomed.sld.cu | Medical applications | medicine | Skull implants (plastic not esp), vertebra (Ti) and heel (Ti) | ||||
| 2017 | ME3 | Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences | Bethany C. Gross, Jayda L. Erkal, Sarah Y. Lockwood, Chengpeng Chen, and Dana M. Spence* | 2014 | Analytical chemistry, January 16, 2014 | Medical applications | medicine | Bone printing | ||||
| 2017 | ME4 | From the printer: Potential of three-dimensional printing for orthopaedic applications | Sze-Wing Mok a,b, Razmara Nizak c, Sai-Chuen Fu a,b, Ki-Wai Kevin Ho a,b, Ling Qin a,b, Danie¨l B.F. Saris c,d, Kai-Ming Chan a,b, Jos Malda | 2014 | Journal of Orthopaedic Translation (2016) 6, 42e49 | Medical applications | medicine | Orthopedic applications | ||||
| 2017 | ME5 | Three-Dimensional Printing of Carbamazepine Sustained-Release Scaffold | Seng Han Lim, Samuel Ming Yuan Chia, Lifeng Kang, Kevin Yi-Lwern Yap | 2016 | Journal of Pharmaceutical Sciences 105 (2016) 2155-2163 | Medical applications | medicine | Drug printing | ||||
| 2017 | ME6 | 3D printing to simulate laparoscopic choledochal surgery | Oliver C. Burdall, Erica Makin, Mark Davenport, Niyi Ade-Ajayi ⁎ | 2016 | Journal of Pediatric Surgery 51 (2016) 828–831 | Medical applications | medicine | surgical procedure simulation | ||||
| 2017 | ME7 | Fabrication of a highly ordered hierarchically designed porous nanocomposite via indirect 3D printing: Mechanical properties and in vitro cell responses | E. Tamjid a,⁎, A. Simchi b,c | 2015 | Materials and Design 88 (2015) 924–931 | Medical applications | medicine | axes and cell growth | ||||
| 2017 | ME8 | Fabrication of scalable tissue engineering scaffolds with dual-pore microarchitecture by combining 3D printing and particle leaching | Soumyaranjan Mohanty a, Kuldeep Sanger a, Arto Heiskanen a, Jon Trifol b, Peter Szabo b, Marin Dufva a, Jenny Emnéus a, AndersWolff a,⁎ | 2016 | Materials Science and Engineering C 61 (2016) 180–189 | Medical applications | medicine | axes and cell growth | ||||
| 2017 | ME9 | Effect of layer printing delay on mechanical properties and dimensional accuracy of 3D printed porous prototypes in bone tissue engineering | Arghavan Farzadia,n, VicknesWaranb, MehranSolati-Hashjina, ZainalAriffAbdulRahmanc, Mitra Asadia, NoorAzuanAbuOsmana | 2015 | Ceramics International41(2015)8320–8330 | Medical applications | medicine | Bone printing | ||||
| 2017 | ME10 | Powder-based 3D printing for bone tissue engineering | G. Brunello a, S. Sivolella a,⁎, R. Meneghello b, L. Ferroni c, C. Gardinc, A. Piattelli d, B. Zavanc,⁎, E. Bressana | 2016 | Biotechnology Advances xxx (2016) xxx–xxx | Medical applications | medicine | Bone printing | ||||
| 2017 | ME11 | Modulation, functionality, and cytocompatibility of three-dimensional printing materials made from chitosan-based polysaccharide composites | Chin-San Wu | 2016 | Materials Science and Engineering C 69 (2016) 27–36 | Medical applications, manufacturing, Multimaterial | medicine | Biocompatibility, Multimaterials, additives | ||||
| 2017 | ME12 | Understanding Spatially Complex Segmental and Branch Anatomy Using 3D Printing: Liver, Lung, Prostate, Coronary Arteries, and Circle of Willis | Ramin Javan, MD, Douglas Herrin, BS, Ardalan Tangestanipoor, MD | 2016 | Academic Radiology, Vol ■, No ■, ■■ 2016 | Medical applications | medicine | tissue printing | ||||
| 2017 | ME13 | Cerebral Aneurysm Clipping Surgery Simulation Using Patient-Specific 3D Printing and Silicone Casting | Justin R. Ryan1,2, Kaith K. Almefty3, Peter Nakaji3, David H. Frakes1,2,4 | 2016 | WORLD NEUROSURGERY 88: 175-181, APRIL 2016 | Medical applications | medicine | surgical procedure simulation | ||||
| 2017 | ME14 | Using 3D Printing to Create Personalized Brain Models for Neurosurgical Training and Preoperative Planning | Caitlin C. Ploch1, Chris S.S.A. Mansi3, Jayaratnam Jayamohan4, Ellen Kuhl1,2 | 2016 | WORLD NEUROSURGERY 90: 668-674, JUNE 2016 | Medical applications | medicine | surgical procedure simulation | ||||
| 2017 | ME15 | 3D printing in Neurosurgery | Francesco Tomasello, Alfredo Conti, Domenico La Torre | 2016 | WORLD NEUROSURGERY -: ---, MONTH 2016 | Medical applications | medicine | surgical procedure simulation | ||||
| 2017 | ME16 | Design and 3D Printing of Scaffolds and Tissues | Jia An, Joanne Ee Mei Teoh, Ratima Suntornnond, Chee Kai Chua* | 2015 | Engineering 2015, 1(2): 261–268 | Medical applications | medicine | Printing and Bone Impression | ||||
| 2017 | ME17 | Process Planning for the Fuse Deposition Modeling of Ankle-Foot-Othoses | Yuan Jina,b* , Yong Heb, Albert Shiha,c | 2016 | Procedia CIRP 42 ( 2016 ) 760 – 765 | Medical applications | medicine | orthosis | ||||
| 2017 | ME18 | Three-dimensional printing technique assisted cognitive fusion in targeted prostate biopsy | Yan Wang a, Xu Gao a, Qingsong Yang b, Haifeng Wang a, Ting Shi a, Yifan Chang a, Chuanliang Xu a, Yinghao Sun a,* | 2015 | Asian Journal of Urology (2015) 2, 214e219 | Medical applications | medicine | surgical procedure simulation | ||||
| 2017 | ME19 | Application of 3D Printing in Medical Simulation and Education | Carling L. Cheung, Nikoo R. Saber | 2016 | Bioengineering for Surgery ISBN 978-0-08-100123-3 | Medical applications | medicine | surgical procedure simulation | ||||
| 2017 | ME20 | 3D printing of polyurethane biomaterials | K.-C. Hung1, C.-S. Tseng2, S.-H. Hsu1,* | 2016 | Advances in Polyurethane Biomaterials. http://dx.doi.org/10.1016/B978-0-08-100614-6.00005-6 Copyright © 2016 Elsevier Ltd. All rights reserved. | Medical applications | medicine | Biocompatibility | ||||
| 2017 | ME21 | Applications of 3D Printing in Cell Biology | 2016 | Cell Biology. http://dx.doi.org/10.1016/B978-0-12-801853-8.00002-8 Copyright © 2016 Elsevier Inc. All rights reserved | Medical applications | medicine | axes and cell growth | |||||
| 2017 | ME22 | A preliminary investigation into the development of 3-D printing of prosthetic sockets | Nicholas Herbert, David Simpson, William D. Spence, William Ion | 2005 | Journal of Rehabilitation Research & Development Volumen 42, Number 2, Pages 141-146, March/April 2005 | Medical applications | medicine | prosthesis | ||||
| 2017 | ME23 | DISEÑO DE UNA PRÓTESIS DE PIERNA PARA AMPUTADOS TRANSTIBIALES | ALEJANDRO JOSÉ DOBERTI MARTÍNEZ, VIVIANA MERUANE NARANJO | 2015 | TESIS DE GRADO, UNIVERSIDAD DE CHILE FACULTAD DE CIENCIAS FÍSICAS Y MATEMÁTICAS DEPARTAMENTO DE INGENIERÍA MECÁNICA, 2015 | Medical applications | medicine | prosthesis | ||||
| 2017 | ME24 | Fabrication of low cost soft tissue prostheses with the desktop 3D printer | Yong He1,2, Guang-huai Xue1,2 & Jian-zhong Fu1,2 | 2014 | SCIENTIFIC REPORTS | 4 : 6973 | DOI: 10.1038/srep06973 | Medical applications | medicine | prosthesis | ||||
| 2017 | ME25 | Fused deposition modeling of patient-specific polymethylmethacrylate implants | David Espalin, Karina Arcaute, David Rodriguez, Francisco Medina, Matthew Posner, Ryan Wicker | 2010 | Rapid Prototyping Journal, Vol. 16 Issue: 3,pp. 164-173 | Medical applications | medicine | skull implants | ||||
| 2019 | ME26 | Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications | Ataee, A., Li, Y., Fraser, D., Song, G., Wen, C. | 2018 | Materials and Design 137, pp. 345-354 | Medical applications | medicine | Bone implants, optimization (lattice), mechanics | ||||
| 2019 | ME27 | Additive manufacturing applications in orthopaedics: A review | Javaid, M., Haleem, A. | 2018 | Journal of Clinical Orthopaedics and Trauma 9(3), pp. 202-206 | Medical applications | medicine | orthopedics | ||||
| 2019 | ME28 | 3D printing and modelling of customized implants and surgical guides for non-human primates | Chen, X., Possel, J.K., Wacongne, C., (...), Klink, P.C., Roelfsema, P.R. | 2017 | Journal of Neuroscience Methods 286, pp. 38-55 | Medical applications | medicine | Implants and guides for surgery | ||||
| 2019 | ME29 | 3D printing and its applications in orthopaedic trauma: A technological marvel | Lal, H., Patralekh, M.K. | 2018 | Journal of Clinical Orthopaedics and Trauma 9(3), pp. 260-268 | Medical applications | medicine | orthopedics | ||||
| 2019 | ME30 | Industry 5.0 and its applications in orthopaedics | Abid Haleem and Mohd Javaid | 2018 | Journal of Clinical Orthopaedics and Trauma | Medical applications | medicine | orthopedics | ||||
| 2019 | ME31 | Additive manufacturing applications in cardiology: A review | Haleem, A., Javaid, M., Saxena, A. | 2018 | Egyptian Heart Journal 70(4), pp. 433-441 | Medical applications | medicine | cardiology | ||||
| 2019 | ME32 | Effects of socket size on metrics of socket fit in trans-tibial prosthesis users | Sanders, J.E., Youngblood, R.T., Hafner, B.J., (...), Ciol, M.A., Allyn, K.J. | 2017 | Medical Engineering and Physics 44, pp. 32-43 | Medical applications | medicine | PROSTHESIS (NOT AM) | ||||
| 2019 | ME33 | Additive manufacturing applications in medical cases: A literature based review | Mohd. Javaid and Abid Haleem | 2018 | Alexandria Journal of Medicine | Medical applications | medicine | CASE STUDIES | ||||
| 2019 | ME34 | Production of customized hip stem prostheses - A comparison between conventional machining and electron beam melting (EBM) | Cronskär, M., Bäckström, M., Rännar, L.-E. | 2013 | Rapid Prototyping Journal 19(5),17093925, pp. 365-372 | Medical applications | Manufacturing, environment, design, medicine | prosthesis | ||||
| 2021 | ME35 | Three-dimensional printing surgical instruments: are we there yet? | Timothy M. Rankin and Nicholas A. Giovinco and Daniel J. Cucher and George Watts and Bonnie Hurwitz and David G. Armstrong | 2014 | J Surg Res. 2014 June 15; 189(2): 193–197. doi:10.1016/j.jss.2014.02.020. | Medical applications | medicine | instruments | ||||
| 2021 | ME36 | 3D Printing of Surgical Instruments for Long-Duration Space Missions | Julielynn Y. Wong and Andreas C. Pfahnl | 2014 | Aviation, Space, and Environmental Medicine x Vol. 85, No. 7 x July 2014 | Medical applications | medicine | instruments | ||||
| 2021 | ME37 | Nuevas Tecnologías para la Sanidad Militar | Crego Vita DM.1, García Cañas R.2, Areta Jiménez FJ.3 | 2017 | Sanidad mil. 2017; 73 (1): 28-30, ISSN: 1887-8571 | Medical applications | medicine | instruments | ||||
| 2021 | ME38 | Three-dimensional printing in surgery: a review of current surgical applications | Hammad H. Malik and Alastair R.J. Darwood and Shalin Shaunak and Priyantha Kulatilake and Abdulrahman A. El-Hilly and Omar Mulki and Aroon Baskaradas | 2015 | j ournal of s u r g i c a l re s e a r c h 1 9 9 ( 2 0 1 5 ) 5 1 2 -5 2 2 | Medical applications | medicine | Anatomical models: surgical planning, education and training; Surgical instruments: preoperative planning, intraoperative use; Implants and prostheses: organ and tissue printing. | ||||
| 2021 | ME39 | The use of three-dimensional printing technology in orthopaedic surgery: A review | Tak Man Wong and Jimmy Jin and Tak Wing Lau and Christian Fang and Chun Hoi Yan and Kelvin Yeung and Michael To and Frankie Leung | 2017 | Journal of Orthopaedic Surgery Volume: 25(1) 1–7 ª Journal of Orthopaedic Surgery 2017 | Medical applications | medicine | Surgical planning, manufacturing of specific instruments for patients, implants, engineering of bone tissues. | ||||
| 2021 | ME40 | Computer-assisted mosaic arthroplasty using patient-specific instrument guides | Manuela Kunz and Stephen D. Waldman and John F. Rudan and Davide D. Bardana and A. James Stewart | 2012 | Knee Surg Sports Traumatol Arthrosc (2012) 20:857–861 | Medical applications | medicine | custom instruments | ||||
| 2021 | ME41 | 3D printing in dentistry | A. Dawood and B. Marti Marti and V. Sauret-Jackson and A. Darwood | 2015 | BRITISH DENTAL JOURNAL VOLUME 219 NO. 11 DEC 11 2015 | Medical applications | medicine | Medical models, drilling and cutting guides, crowns and dentures, dental models for restorative dentistry, digital orthodontics, dental implants, maxillofacial implants, instruments. | ||||
| 2021 | ME42 | Manufacture and evaluation of 3-dimensional printed sizing tools for use during intraoperative breast brachytherapy | Joshua M. Walker and David A. Elliott and Charlotte D. Kubicky and Charles R. Thomas and Arpana M. Naik | 2016 | Journal Article published Apr 2016 in Advances in Radiation Oncology volume 1 issue 2 on pages 132 to 135 | Medical applications | medicine | instrument | ||||
| 2021 | ME43 | 3D Printed Surgical Instruments: The Design and Fabrication Process | Mitchell George and Kevin R. Aroom and Harvey G. Hawes and Brijesh S. Gill and Joseph Love | 2016 | Journal Article published Jan 2017 in World Journal of Surgery volume 41 issue 1 on pages 314 to 319 | Medical applications | medicine | instrument | ||||
| 2021 | ME44 | On Demand Additive Manufacturing of a Basic Surgical Kit | Shayne Kondor and CAPT Gerald Grant and Peter Liacouras and MAJ James R. Schmid and LTC Michael Parsons and Vipin K. Rastogi and Lisa S. Smith and Bill Macy and Brian Sabart and Christian Macedonia | 2013 | Journal Article published 1 Sep 2013 in Journal of Medical Devices volume 7 issue 3 | Medical applications | medicine | instrument | ||||
| 2021 | ME45 | ISO 11138-1-Sterilization of health care products — Biological indicators — Part 1: General requirements | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2017 | Medical applications | medicine | norms | |||||
| 2021 | ME46 | MANUAL DE BIOSEGURIDAD EN EL LABORATORIO | ORGANIZACIÓN MUNDIAL DE LA SALUD | 2005 | Medical applications | medicine | norms | |||||
| 2021 | ME47 | NORMA TÉCNICA COLOMBIANA NTC 4426-3 ESTERILIZACIÓN DE PRODUCTOS PARA EL CUIDADO DE LA SALUD. INDICADORES BIOLÓGICOS. PARTE 3: INDICADORES BIOLÓGICOS PARA PROCESOS DE ESTERILIZACIÓN CON CALOR HÚMEDO. | Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC) | 2016 | Medical applications | medicine | norms | |||||
| 2021 | ME48 | Material issues in additive manufacturing: A review | Singh, S., Ramakrishna, S., Singh, R. | 2017 | Journal of Manufacturing Processes 25, pp. 185-200 | manufacturing, medicine | manufacturing, medicine | Applied materials to fabrics | ||||
| 2021 | ME49 | TECHNICAL APPLICATION GUIDE: Data Segmentation for Medical 3D Printing | STRATASYS | 2016 | STRATASYS | manufacturing, medicine | manufacturing, medicine | digital images | ||||
| 2021 | ME50 | A Simple 3-Dimensional Printed Aid for a Corrective Palmar Opening Wedge Osteotomy of the Distal Radius | Philipp Honigmann and Florian Thieringer and Regula Steiger and Mathias Haefeli and Ralf Schumacher and Julia Henning | 2016 | Journal Article published Mar 2016 in The Journal of Hand Surgery volume 41 issue 3 on pages 464 to 469 | Medical applications | medicine | osteotomy, palmar, hand, specific tool or instrument for patient | ||||
| 2021 | ME51 | 3D templating and patient-specific cutting guides (Knee-Plan®) in total knee arthroplasty: Postoperative CT-based assessment of implant positioning | J.-P. Franceschi and A. Sbihi | 2014 | Orthopaedics & Traumatology: Surgery & Research 100 (2014) S281–S286 | Medical applications | medicine | osteoarthritis, knee, specific tool or instrument for patient, planning, software, knee arthroplasty | ||||
| 2021 | ME51 A | Knee-Plan®system, Symbios Orthopédie SA | SYMBIOS | 2014, 2021 | https://symbios.ch/en/medical-professionals/products-and-solutions/knee-plan/?lang=en | Medical applications | medicine | osteoarthritis, knee, specific tool or instrument for patient, planning, software, knee arthroplasty | ||||
| 2021 | ME52 | Computer-Assisted Planning and Three-Dimensional-Printed Patient-Specific Instrumental Guide for Corrective Osteotomy in Post-Traumatic Femur Deformity: A Case Report and Literature Review | Lau Chi-Kay and Chui King-him and Lee Kin-bong and Li Wilson | 2018 | Journal of Orthopaedics, Trauma and Rehabilitation 24 (2018) 12e17 | Medical applications | medicine | Lower limb osteotomy, specific tool or instrument for the patient. | ||||
| 2021 | ME53 | Three-dimensional printing in spine surgery: a review of current applications | Yixuan Tong and Daniel James Kaplan and Jeffrey M. Spivak and John A. Bendo | 2020 | The Spine Journal 20 (2020) 833−846 | Medical applications | medicine | SURGERY OF THE SPINE, state of the art | ||||
| 2021 | ME54 | Biomedicine | Chao Lin | 2012 | InTech | Medical applications | medicine | state of the art. | ||||
| 2021 | ME55 | Additive Manufacturing Solutions for Improved Medical Implants | Vojislav Petrovic and Juan Vicente and Jose Ramn and Luis Portols | 2012 | InTech, BOOK BIOMEDICINE | Medical applications | medicine | state of the art. | ||||
| 2021 | ME56 | Polymer-Based Additive Manufacturing | Declan M. Devine | 2019 | Springer International Publishing | Medical applications | medicine | state of the art. | ||||
| 2021 | ME57 | Current Market for Biomedical Implants | Aleksandra Foerster and Laura Ruiz Cantu and Ricky Wildman and Christopher Tuck | 2019 | Springer International Publishing, Book Polymer-Based Additive Manufacturing | Medical applications | medicine | state of the art. | ||||
| 2021 | ME58 | PHONAK | PHONAK | 2021 | https://www.phonak.com/us/en.html | Medical applications | medicine | customization, headphone, ear implant | ||||
| 2021 | ME59 | INVISALIGN | PERFECT SMILE | 2021 | https://perfect-smile.cz/rovnatka-invisalign?gclid=EAIaIQobChMIgJKo8rOl6gIVzJ6zCh3augAeEAAYASAAEgI4avD_BwE | Medical applications | medicine | customization, frenulum, dental implant | ||||
| 2021 | ME60 | ENVISIONTEC | ENVISIONTEC | 2021 | https://envisiontec.com/3d-printing-industries/medical/ | Medical applications | medicine | Biocompatible materials for auditory and dental/orthodontic implants. | ||||
| 2021 | ME61 | ISO STANDARD · ISO 10993-1 Biological evaluation of medical devices. Part 1: Evaluation and testing within a risk management process | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2018 | INTERNATIONAL STANDARD ORGANIZATION-ISO | Medical applications | medicine | norms | ||||
| 2021 | ME62 | ISO STANDARD · ISO 10993-4 Biological evaluation of medical devices —Part 4: Selection of tests for interactions with blood | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2009 | INTERNATIONAL STANDARD ORGANIZATION-ISO | Medical applications | medicine | norms | ||||
| 2021 | ME63 | ISO STANDARD · ISO 10993-5 Biological evaluation of medical devices —Part 5: Tests for in vitro cytotoxicity | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2009 | INTERNATIONAL STANDARD ORGANIZATION-ISO | Medical applications | medicine | norms | ||||
| 2021 | ME64 | ISO 10993-11:2009 Biological evaluation of medical devices - Part 11: Tests for systemic toxicity (ISO 10993-11:2006) | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2009 | INTERNATIONAL STANDARD ORGANIZATION-ISO | Medical applications | medicine | norms | ||||
| 2021 | ME65 | ISO 10993-18:2005 Biological evaluation of medical devices — Part 18: Chemical characterization of materials | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2009 | INTERNATIONAL STANDARD ORGANIZATION-ISO | Medical applications | medicine | norms | ||||
| 2021 | ME66 | ISO 10993-17:2002 Biological evaluation of medical devices — Part 17: Establishment of allowable limits for leachable substances | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2009 | INTERNATIONAL STANDARD ORGANIZATION-ISO | Medical applications | medicine | norms | ||||
| 2021 | ME67 | ISO 10993-3:2014 Biological evaluation of medical devices — Part 3: Tests for genotoxicity, carcinogenicity and reproductive toxicity | INTERNATIONAL STANDARD ORGANIZATION-ISO | 2009 | INTERNATIONAL STANDARD ORGANIZATION-ISO | Medical applications | medicine | norms | ||||
| 2021 | ME68 | DECLARATION OF COMPLIANCE WITH EN ISO 10993-1 Overall Biological Risk Assessment for the 3-D Printing Material Ultem 1010 | Dieter R. Dannhorn, Erwin Deiringer | 2018 | Stratasys GmbH. Airport Boulevard B120, 77836 Rheinmϋnster Germany | Medical applications | medicine | Cytotoxicity, Irritation, Delayed-type Hypersensitivity, Material-mediated Pyrogenicity, Acute Systemic Toxicity, Chemical Characterization, Permissible Limits for Leachable Substances, Compliance with ULTEM Standards | ||||
| 2021 | ME69 | Filamentos BioCompatibles | IMKR.COM, Filaments.CA, | 2020, 2021 | https://www.imakr.com/pcl-filaments-for-3d-printers, https://filaments.ca/products/pcl-low-temperature-filament-natural-1-75mm#:~:text=Our%20PCL%20(Polycaprolactone)%203D%20filament,such%20as%20PLA%2C%20ABS%20etc. | Medical applications | medicine | Biocompatible filaments, semi-permanent implants. | ||||
| 2021 | ME70 | Hearing aids | STARKEY | 2020, 2021 | https://www.starkey.co.uk/hearing-aids | Medical applications | medicine | customization, headphone, ear implant | ||||
| 2021 | ME71 | FIGURE PRINTS WORLD OF WARCRAFT | SQUIP | 2020, 2021 | https://squip.com/product/wow/ | Medical applications | customization | customization, action figures | ||||
| 2021 | ME72A | Custom 3D Printed Glasses Glasses that fit you. Only you. | SPECSY | 2020, 2021 | https://home.specsy.com/ | Medical applications | customization | personalized glasses | ||||
| 2021 | ME72B | custom-made-3d-printed-glasses | 3D BROOKLIN | 2020 | https://3dbrooklyn.com/custom-made-3d-printed-glasses | Medical applications | customization | personalized glasses | ||||
| 2021 | ME73A | THE EARTH SHOE | QUERENCIA STUDIO | https://www.querenciastudio.com/products/the-earth-shoe | Medical applications | customization | Custom footwear, custom shoes | |||||
| 2021 | ME73B | HEROES SANDAL | HEROES SANDALS | https://www.heroessandals.com/howtomeasure | Medical applications | customization | Custom footwear, custom shoes | |||||
| 2021 | ME73C | FOOTB 3D CUSTOM INSOLE | CASCA | https://casca.com/products/footb3d-custom-insole | Medical applications | customization | Custom footwear, custom shoes | |||||
| 2021 | ME73D | PLANTILLAS PERSONALIZADAS DE WIIVV | wiivv | https://wiivv.com/pages/insoles | Medical applications | customization | Custom footwear, custom shoes | |||||
| 2021 | ME74 | Personalized Surgical Instruments | Shayne Kondor, CAPT Gerald Grant, Peter Liacouras, MAJ James R. Schmid, LTC Michael Parsons, Bill Macy, Brian Sabart, Christian Macedonia | 2013 | Journal of Medical Devices, SEPT VOL 7 | Medical applications | medicine | instrument | ||||
| 2021 | ME75 | Cardiovascular Three-Dimensional Printing in Non-Congenital Percutaneous Interventions | Manuel de Oliveira-Santos, Eduardo Oliveira-Santos, Lino Gonçalves, João Silva Marques | 2019 | Journal Article published Oct 2019 in Heart, Lung and Circulation volume 28 issue 10 on pages 1525 to 1534 | Medical applications | medicine | instrument | ||||
| 2021 | ME76 | 3D Printed Surgical Instruments: The Design and Fabrication Process | Mitchell George and Kevin R. Aroom and Harvey G. Hawes and Brijesh S. Gill and Joseph Love | 2016 | World J Surg. 2017 January ; 41(1): 314–319 | Medical applications | medicine | instrument | ||||
| 2021 | ME77 | Diseño y Construcción de Prótesis de Miembros Superiores e Inferiores mediante Impresión 3D para Personas Discapacitadas de Bajos Recursos | Roberto Algarín Roncallo, Javier Vargas Duque, Luis López Taborda, Guadalupe Avelar, Milena Mendoza, Ramiro Rodríguez Márceles | 2015 | Proyecto de Investigación, desarrollo e innovación 3D INGENIERIA BQ SAS, CE CAMILO | Medical applications | medicine | prosthesis | ||||
| 2021 | ME78 | Experimental characterization and theoretical modelling of the mechanical behaviour of ABS in the 3D printing process | Roberto Algarín, Luis López, Diego Guillen, William Fuentes | 2016-2021 | Proyecto de Aula Doctoral UNIVERSIDAD DEL NORTE/Proyecto de investigación, desarrollo e innovación 3D INGENIERIA BQ SAS, artículo científico no publicado | Medical applications, Mechanical modeling, Failure theory | medicine, mechanics, failure theory | prosthesis, mechanical characterization, simulation, failure theory | ||||
| 2021 | ME79 | Prótesis electromecánicas de miembro inferior y superior para personas amputadas de bajos recursos | Roberto Algarín Roncallo, Javier Vargas, Luis López, Guadalupe Avelar, Diego Serrano Bula | 2017 | Proyecto de Investigación, desarrollo e innovación 3D INGENIERIA BQ SAS, CE CAMILO Y UNIVERSIDAD AUTONOMA DEL CARIBE (solo protesis superior electromecánica) | Medical applications | medicine | prosthesis | ||||
| 2021 | ME80 | Diseño Industrial de cubiertas cosméticas y personalizadas para prótesis de miembros inferiores. | Roberto Algarín Roncallo, Javier Vargas, Libardo Reyes, Estudiantes de asignatura de diseño industrial, Luis López. | 2017 | Proyecto de Aula de Pregrado UNIVERSIDAD DEL NORTE/ Proyecto de Investigación, desarrollo e innovación 3D INGENIERIA BQ SAS | Medical applications | medicine | prosthesis | ||||
| 2021 | ME81 | Diseño y Fabricación de cubiertas cosméticas para prótesis de miembros inferiores. | Roberto Algarín Roncallo, Javier Vargas, Luis López | 2018 | Proyecto de Investigación, desarrollo e innovación 3D INGENIERIA BQ SAS | Medical applications, Mechanical modeling | medicine, mechanics | prosthesis, mechanical characterization | ||||
| 2021 | ME82 | Elementos protésicos de fácil acceso para personas con amputación de miembro inferior | Roberto Algarín Roncallo, Javier Vargas Duque, Luis López Taborda | 2019 | Proyecto de Investigación, desarrollo e innovación 3D INGENIERIA BQ SAS, SENA Y UNIVERSIDAD DEL ATLANTICO (Solo pruebas en elementos protesicos) | Medical applications, Mechanical modeling, Failure theory | medicine, mechanics, failure theory | prosthesis, mechanical characterization, simulation, failure theory | ||||
| 2021 | ME83 | IMPLEMENTACIÓN DE CUÑA Y DESARROLLO DE HERRAMIENTA INFORMÁTICA APLICADA A PROCESOS DE OSTEOTOMÍA UTILIZANDO TECNOLOGÍA FDM (MODELADO DE DEPOSICIÓN FUNDIDA): UN CASO CLÍNICO. | MEZA BALZA SAIN JOSÉ, PÉREZ PIZARRO RAFAEL JOSÉ | 2018 | UNIVERSIDAD DEL ATLÁNTICO, FACULTAD DE INGENIERÍA DEPARTAMENTO DE INGENIERÍA PROGRAMA DE INGENIERÍA MECÁNICA | Medical applications, Mechanical modeling | medicine, mechanics | Wedge for lower limb osteotomy, mechanical characterization, simulation. | ||||
| 2021 | ME84 | DISEÑO Y CONSTRUCCIÓN DE PROTOTIPO DE MOLDE PARA RECONSTRUCCIÓN ÓSEA A PARTIR DE TOMOGRAFÍA COMPUTARIZADA MEDIANTE IMPRESIÓN 3D | MARULANDA HERRERA HÉCTOR, MIGUEL ORTIZ LLANOS ANDRÉS FELIPE | 2021 | UNIVERSIDAD DEL ATLÁNTICO FACULTAD DE INGENIERÍA DEPARTAMENTO DE INGENIERÍA PROGRAMA DE INGENIERÍA MECÁNICA | Medical applications, Mechanical modeling | medicine, mechanics | Mold for bone implant, mechanical characterization, simulation. | ||||
| 2021 | ME85 | IMPLEMENTACIÓN DE UN PROTOTIPO DE SEPARADOR AUTOESTÁTICO PARA ABDOMINOPLASTIA | DAVID RICARDO ESCALANTE MEJÍAJOSÉ ALEJANDRO LAMADRID LEMUS | 2021 | UNIVERSIDAD DEL ATLÁNTICO FACULTAD DE INGENIERÍA PROGRAMA DE INGENIERÍA MECÁNICA | Medical applications, Mechanical modeling | medicine, mechanics | Prototype surgical instrument/tool, mechanical characterization, simulation. | ||||
| 2021 | ME86 | Computer Aided Design of Large-Format Prefabricated Cranial Plates | David Dean, Kyoung-June Min, Angus Bond | 2003 | THE JOURNAL OF CRANIOFACIAL SURGERY / VOLUME 14, NUMBER 6 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME87 | Customized Cranioplasty Implants Using Three-Dimensional Printers and Polymethyl-Methacrylate Casting | Bum-Joon Kim, Ki-Sun Hong,Kyung-Jae Park, Dong-Hyuk Park, Yong-Gu Chung, Shin-Hyuk Kang | 2012 | J Korean Neurosurg Soc 52 December 2012 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME88 | Cold-Injection Molded Gentamicin-Impregnated Polymethyl Methacrylate Implants for Cranioplasty | Mena Mekhael Fahem, Nabeel Hameed Ali, Joseph Ravindra Duddu, Harleen Luther | 2021 | Journal Article published 29 Jul 2021 in Operative Neurosurgery | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME89 | Customized Cost‑Effective Polymethyl‑Methacrylate Cranioplasty Implant Using Three‑Dimensional Printer | Sambardhan Dabadi, Raju Raj Dhungel, Upama Sharma, Dinuj Shrestha, Pritam Gurung, Resha Shrestha, Basant Pant | 2021 | Asian Journal of Neurosurgery | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME90 | Cranioplasty with preoperatively customized Polymethyl-methacrylate by using 3-Dimensional Printed Polyethylene Terephthalate Glycol Mold | Mehmet Beşir Sürme, Omer Batu Hergunsel, Bekir Akgun and Metin Kaplan | 2018 | Journal of Neuroscience and Neurological Disorders | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME91 | Customized Polymethylmethacrylate Cranioplasty Implants Using 3-Dimensional Printed Polylactic Acid Molds: Technical Note with 2 Illustrative Cases | Joe Abdel Hay, Tarek Smayra, Ronald Moussa | 2017 | WORLD NEUROSURGERY | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME92 | Cost-Effective Technique of Fabrication of Polymethyl Methacrylate Based Cranial Implant Using Three-Dimensional Printed Moulds and Wax Elimination Technique | Jimish B. Desai | 2019 | The Journal of Craniofacial Surgery Volume 30, Number 4, June 2019 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME93 | Low-Cost Customized Cranioplasty with Polymethyl Methacrylate Using 3D Printer Generated Mold: An Institutional Experience and Review of Literature | Ankit Chaudhary, Virendra Deo Sinha, Sanjeev Chopra, Jitendra Shekhawat, Gaurav Jain | 2020 | Indian Journal of Neurotrauma Vol. 17 No. 2/2020 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME94 | Comparison between autologous bone grafts and acrylic (PMMA) implants – A retrospective analysis of 286 cranioplasty procedures | G.H. Vince, J. Kraschl, H. Rauter, M. Stein, S. Grossauer, E. Uhl | 2019 | Journal of Clinical Neuroscience 61 (2019) 205–209 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME95 | Alloplastic Cranioplasty Reconstruction A Systematic Review Comparing Outcomes With Titanium Mesh, Polymethyl Methacrylate, Polyether Ether Ketone, and Norian Implants in 3591 Adult Patients | Jeremie D. Oliver, Joseph Banuelos, Amjed Abu-Ghname, Krishna S. Vyas, and Basel Shara | 2019 | Annals of Plastic Surgery • Volume 82, Supplement 4, May 2019 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME96 | Traumatic Fracture of a Polymethyl Methacrylate Patient-Specific Cranioplasty Implant | Andrew L. Ko, John D. Nerva, Jason J. J. Chang, Randall M. Chesnut | 2014 | WORLD NEUROSURGERY 82 [3/4]: 536.e11-536.e13, SEPTEMBER/OCTOBER 2014 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME97 | Outcome in patient-specific PEEK cranioplasty: A two-center cohort study of 40 implants | J. Jonkergouw, S.E.C.M. van de Vijfeijken, E. Nout, T. Theys, E. Van de Casteele, H. Folkersma, P.R.A.M. Depauw, A.G. Becking | 2016 | Journal of Cranio-Maxillo-Facial Surgery 44 (2016) 1266-1272 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME98 | 3D-Printer-Assisted Patient-Specific Polymethyl Methacrylate Cranioplasty: A Case Series of 16 Consecutive Patients | Stephan N. Schon, Nicolas Skalicky, Neha Sharma, Daniel W. Zumofen, Florian M. Thieringer | 2021 | World Neurosurg. (2021) 148:e356-e362 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME99 | Rehabilitation of a cranial defect with a preoperatively customized polymethyl-methacrylate prosthesis using 3-dimensional printed p olylactic acid mold: A case report | Anita Kapri, Pushpa Kumari, Gulnar Sethna | 2020 | IP Annals of Prosthodontics and Restorative Dentistry 2020;6(2):105–109 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME100 | Long-Term Complications of Cranioplasty Using Stored Autologous Bone Graft, Three- Dimensional Polymethyl Methacrylate, or Titanium Mesh After Decompressive Craniectomy: A Single-Center Experience After 596 Procedures | Mun-Chun Yeap, Po-Hsun Tu, Zhuo-Hao Liu, Po-Chuan Hsieh, Yu-Tse Liu, Ching-Yi Lee, Hung-Yi Lai, Chun-Ting Chen, Yin-Cheng Huang, Kuo-chen Wei, Chieh-Tsai Wu, Ching-Chang Chen | 2019 | WORLD NEUROSURGERY 128: e841-e850, AUGUST 2019 | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME101 | BIOCOMPATIBILITY STUDIES ON SILICONE RUBBER | P.V. Mohanan and K. Rathinam | 1995 | Proceedings RC IEEE-EMBS & 14th BMESI - 1995 | medical applications | medicine | Biocompatible materials for implants | ||||
| 2021 | ME102 | A classification of cranial implants based on the degree of difficulty in computer design and manufacture | Jules Poukens Paul Laeven Maikel Beerens Gerard Nijenhuis Jos Vander Sloten Paul Stoelinga Peter Kessler | 2008 | THE INTERNATIONAL JOURNAL OF MEDICAL ROBOTICS AND COMPUTER ASSISTED SURGERY Int J Med Robotics Comput Assist Surg 2008; 4: 46–50. | medical applications | medicine | bone implant, customization | ||||
| 2021 | ME103 | Mechanical performances of hip implant design and fabrication with PEEK composite | Bankole I. Oladapo, S. Abolfazl Zahedi, Sikiru O. Ismail | 2021 | Polymer 227 (2021) 123865 | medical applications | medicine | Bone implant, hip implant, customization | ||||
| 2021 | ME104 | Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance | S. Berretta, K. Evans, O. Ghita | 2018 | Materials and Design 139 (2018) 141–152 | medical applications | medicine | bone implant, skull implant, customization | ||||
| 2021 | ME105 | Mechanical characterization of biocompatible PEEK by FDM | Yachen Zhao, Kai Zhao, Yuchan Li, Fei Chen | 2020 | Journal of Manufacturing Processes 56 (2020) 28–42 | medical applications | medicine | bone implant, skull implant, customization | ||||
| 2023 | ME106 | Design of Additively Manufactured Structures for Biomedical Applications: A Review of the Additive Manufacturing Processes Applied to the Biomedical Sector | Calignano, F., Galati, M., Iuliano, L., Minetola, P. | 2019 | Journal of Healthcare Engineering, 2019, art. no. 9748212. | medical applications | medicine | - | ||||
| 2023 | ME107 | A Co-Design Method for the Additive Manufacturing of Customised Assistive Devices for Hand Pathologies | Gherardini, F., Mascia, M.T., Bettelli, V., Leali, F. | 2019 | Journal of Integrated Design and Process Science, 22(1), pp. 21-37. | medical applications | medicine | hand orthosis | ||||
| ME108 | A review on 3D printing techniques for medical applications | Mallikarjuna N Nadagouda, Vandita Rastogi and Megan Ginn | 2020 | Current Opinion in Chemical Engineering 2020, 28:152–157 | medical applications | medicine | - | |||||
| ME109 | Medical Applications of Biomaterials: The Case of Design and Manufacture of Orthopedic Corsets Made of Polylactic Acid by Additive Manufacturing | Molnár, I., Morovič, L., Sobrino, D.R.D., Lecký, Š., Michal, D. | 2019 | Materials Science Forum, 952, pp. 223-232. | - | - | - | |||||
| 2017 | FA1 | Additive manufacturing of fatigue resistant materials: Challenges and opportunities | Aref Yadollahi a, Nima Shamsaei b,⇑ | 2017 | International Journal of Fatigue 98 (2017) 14–31 | fatigue modeling | fatigue | state of the art fatigue. | ||||
| 2017 | FA2 | Microstructure Evolution, Tensile Properties, and Fatigue Damage Mechanisms in Ti-6Al-4V Alloys Fabricated by Two Additive Manufacturing Techniques | Yuwei Zhaia,*, Haize Galarragaa, and Diana A. Ladosa | 2015 | Procedia Engineering 114 ( 2015 ) 658 – 666 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA3 | Fatigue behavior of IN718 microtrusses produced via additive manufacturing | Lena Huynh, John Rotella, Michael D. Sangid ⁎ | 2016 | Materials and Design 105 (2016) 278–289 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA4 | Microstructure, static properties, and fatigue crack growth mechanisms in Ti-6Al-4V fabricated by additivemanufacturing: LENS and EBM | Yuwei Zhai ⁎, Haize Galarraga, Diana A. Lados | 2016 | Engineering Failure Analysis 69 (2016) 3–14 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA5 | Fatigue Behaviour of Additively Manufactured Ti-6Al-4V | Amanda Sterlinga, Nima Shamsaeia,b*, Brian Torriesa, Scott M. Thompsona,b | 2015 | Procedia Engineering 133 ( 2015 ) 576 – 589 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA6 | FatigueperformanceevaluationofselectivelasermeltedTi–6Al–4V | P.Edwards a, M.Ramulu b,n | 2014 | Materials Science&EngineeringA598(2014)327–337 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA7 | Fatigue properties of a titanium alloy (Ti–6Al–4V) fabricated via electron beam melting (EBM): Effects of internal defects and residual stress q | Nikolas Hrabe a,⇑, Thomas Gnäupel-Herold b, Timothy Quinn | 2017 | International Journal of Fatigue 94 (2017) 202–210 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA8 | Enhancement of Low-Cycle Fatigue Performance From Tailored Microstructures Enabled by Electron Beam Melting Additive Manufacturing Technology | Philip A. Morton, Jorge Mireles1, Heimdall Mendoza, Paola M. Cordero, Mark Benedict, Ryan B. Wicker | 2015 | Journal of Mechanical Design, NOVEMBER 2015, Vol. 137 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA9 | Defect distribution and microstructure heterogeneity effects on fracture resistance and fatigue behavior of EBM Ti–6Al–4V | Mohsen Seifi a,⇑, Ayman Salem b, Daniel Satko b, Joshua Shaffer b, John J. Lewandowski | 2017 | International Journal of Fatigue 94 (2017) 263–287 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA10 | Effects of Defects in Laser Additive Manufactured Ti-6Al-4V on Fatigue Properties | Eric Wyciska,*, Andreas Solbachb, Shafaqat Siddiquec, Dirk Herzogb, Frank Waltherc, Claus Emmelmanna | 2014 | Physics Procedia 56 ( 2014 ) 371 – 378 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA11 | Fatigue analysis of FDM materials | John Lee, Adam Huang | 2013 | Rapid Prototyping Journal, Vol. 19 Issue: 4, pp.291-299 | fatigue modeling | fatigue | fdm | ||||
| 2017 | FA12 | Fatigue Life of Titanium Alloys Fabricated by Additive Layer Manufacturing Techniques for Dental Implants | KWAI S. CHAN, MARIE KOIKE, ROBERT L. MASON, and TORU OKABE | 2013 | METALLURGICAL AND MATERIALS TRANSACTIONS A, 1010—VOLUME 44A, FEBRUARY 2013 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA13 | Empirical Approach to Understanding the Fatigue Behavior of Metals Made Using Additive Manufacturing | DAVID B. WITKIN, THOMAS V. ALBRIGHT, and DHRUV N. PATEL | 2016 | METALLURGICAL AND MATERIALS TRANSACTIONS A, VOLUME 47A, AUGUST 2016—3823 | fatigue modeling | fatigue | metal | ||||
| 2017 | FA14 | Fatigue Behavior of FDM Parts Manufactured with Ultem 9085 | MATTHIAS FISCHER 1,2,3 and VOLKER SCHOPPNER | 2017 | JOM (Journal of Metals), Vol. 69, No. 3, 2017 | fatigue modeling | fatigue | fdm | ||||
| 2017 | FA15 | FATIGUE CHARACTERIZATION OF 3D PRINTED ELASTOMER MATERIAL | Jacob P. Moore and Christopher B. Williams | 2012 | - | fatigue modeling | fatigue | 3DP Translation in English: 3DP | ||||
| 2017 | FA16 | Material Property Testing of 3D-Printed Specimen in PLA on an Entry-Level 3D Printer | Todd Letcher | 2015 | Proceedings of the ASME 2014 International Mechanical Engineering Congress & Exposition IMECE2014 November 14-20, 2014, Montreal, Quebec, Canada | fatigue modeling | fatigue | fdm | ||||
| 2017 | FA17 | Tensile and fatigue behavior of layered acrylonitrile butadiene styrene | Sophia Ziemian, Maryvivian Okwara, Constance Wilkens Ziemian | 2015 | Rapid Prototyping Journal, Vol. 21 Issue: 3, pp.270-278 | fatigue modeling | fatigue | fdm | ||||
| 2017 | FA18 | Fatigue of injection molded and 3D printed polycarbonate urethane in solution | Andrew T. Miller a, *, David L. Safranski d, Kathryn E. Smith d, Dalton G. Sycks c, Robert E. Guldberg a, b, Ken Gall | 2017 | Polymer 108 (2017) 121e134 | fatigue modeling, manufacturing | fatigue | fff, process chain, multimaterial, additive | ||||
| 2017 | FA19 | Characterization of stiffness degradation caused by fatigue damage of additive manufactured parts | C.W. Ziemian a,⁎, R.D. Ziemianb, K.V. Haile a | 2016 | Materials and Design 109 (2016) 209–218 | fatigue modeling | fatigue | fdm | ||||
| 2017 | FA20 | Fatigue lifespan study of PLA parts obtained by additive manufacturing | R.Jerez-MesaaJ.A.Travieso-RodriguezaJ.Llumà-FuentesaG.Gomez-GrasbD.Puiga | 2017 | Procedia Manufacturing Volume 13, 2017, Pages 872-879 | fatigue modeling | fatigue | fdm | ||||
| 2017 | FA21 | Fatigue performance of fused filament fabrication PLA specimens | Giovanni Gomez-Gras a, Ramón Jerez-Mesa b, J. Antonio Travieso-Rodriguez b,⁎, Jordi Lluma-Fuentes | 2018 | Materials and Design 140 (2018) 278–285 | fatigue modeling | fatigue | fdm | ||||
| 2021 | FA22 | Flexural fatigue properties of polycarbonate fused-deposition modelling specimens | Josep M. Puigoriol-Forcada, Alex Alsina, Antonio G. Salazar-Martín, Giovanni Gomez-Gras, Marco A. Pérez | 2018 | Journal Article published Oct 2018 in Materials & Design volume 155 on pages 414 to 421 | fatigue modeling | fatigue | fdm | ||||
| 2021 | FA23 | Caracterización De Las Probetas De Policarbonato Fabricadas Por FDM Sometidas A Fatiga Por Flexión Rotativa Y Recubiertas Con Resina Epoxi | Samir Alberto Pava Barreto, Kevin Antonio Álvarez López | 2019 | UNIVERSIDAD DEL ATLÁNTICO, FACULTAD DE INGENIERÍA, PROGRAMA DE INGENIERÍA MECÁNICA | fatigue modeling, manufacturing | fatigue | fff, process chain, post-processing, epoxy resin | ||||
| 2021 | FA24 | Fatigue behaviour of FDM-3D printed polymers, polymeric composites and architected cellular materials | Vigneshwaran Shanmugam and Oisik Das and Karthik Babu and Uthayakumar Marimuthu and Arumugaprabu Veerasimman and Deepak Joel Johnson and Rasoul Esmaeely Neisiany and Mikael S. Hedenqvist and Seeram Ramakrishna and Filippo Berto | 2021 | International Journal of Fatigue 143 (2021) 106007 | fatigue modeling | fatigue | state of the art fatigue. | ||||
| 2021 | FA25 | A review of the fatigue behavior of 3D printed polymers | Lauren Safai and Juan Sebastian Cuellar and Gerwin Smit and Amir A. Zadpoor | 2019 | Additive Manufacturing 28 (2019) 87–97 | fatigue modeling | fatigue | state of the art fatigue. | ||||
| 2021 | FA26 | Static and fatigue behaviour of continuous fibre reinforced thermoplastic composites manufactured by fused deposition modelling technique | Alberto D. Pertuz and Sergio Díaz-Cardona and Octavio Andrés González-Estrada | 2020 | International Journal of Fatigue 130 (2020) 105275 | fatigue modeling | fatigue | Fatigue FFF Modified, multimaterial | ||||
| 2017 | E1 | Investigating the feasibility of supply chain-centric business models in 3D chocolate printing: A simulation study | Fu Jia a,b, XiaofengWang c,⁎, Navonil Mustafee a, Liang Hao d | 2016 | Technological Forecasting & Social Change 102 (2016) 202–213 | Market research and environment | environment | economy | ||||
| 2017 | E2 | A global sustainability perspective on 3D printing technologies | MalteGebler,AntonJ.M.SchootUiterkamp,CindyVisser | 2014 | Energy Policy74(2014)158–167 | Market research and environment | environment | environment | ||||
| 2017 | E3 | From rapid prototyping to home fabrication: How 3D printing is changing business model innovation | Thierry Rayna a, Ludmila Striukova b,⁎ | 2016 | Technological Forecasting & Social Change 102 (2016) 214–224 | Market research and environment | environment | economy | ||||
| 2017 | E4 | Analysis of energy utilization in 3d printing processes | Tao Peng | 2016 | Procedia CIRP 40 ( 2016 ) 62 – 67 | Market research and environment | environment | energy | ||||
| 2017 | E5 | An exposure assessment of desktop 3D printing | By Tracy L. Zontek, Burton R. Ogle, John T. Jankovic, Scott M. Hollenbeck | 2016 | J. Chem. Health Safety (2016), JCHAS-902; No of Pages 11 | Market research and environment | environment | health | ||||
| 2017 | E6 | Economic implications of 3D printing:Market structure models in light of additive manufacturing revisited | Christian Weller,RobinKleer n, FrankT.Piller | 2015 | Int. J.ProductionEconomics164(2015)43–56 | Market research and environment | environment | economy | ||||
| 2017 | E7 | Impact of additive manufacturing technology adoption on supply chain management processes and components | Katrin Oettmeier, Erik Hofmann | 2016 | Journal of Manufacturing Technology Management, Vol. 27 Issue: 7,pp. 944-968 | Market research and environment | environment | economy | ||||
| 2017 | E8 | Impact of additive manufacturing on business competitiveness: a multiple case study | Mojtaba Khorram Niaki, Fabio Nonino, | 2017 | Journal of Manufacturing Technology Management, Vol. 28 Issue: 1,pp. 56-74 | Market research and environment | environment | economy | ||||
| 2017 | E9 | Evaluation of Cost Structures of Additive Manufacturing Processes Using a New Business Model | Schröder, M., Falk, B., Schmitt, R. | 2015 | Procedia CIRP 30, pp. 311-316 | Market research and environment | environment | economy | ||||
| 2017 | E10 | Additive manufacturing technology adoption: an empirical analysis of general and supply chain-related determinants | Katrin Oettmeier1 • Erik Hofmann1 | 2017 | J Bus Econ (2017) 87:97–124 | Market research and environment | environment | economy | ||||
| 2017 | E10B | Informing additive manufacturing technology adoption: total cost and the impact of capacity utilisation | Baumers, M., Beltrametti, L., Gasparre, A., Hague, R. | 2017 | International Journal of Production Research pp. 1-14 | Market research and environment | environment | economy | ||||
| 2017 | E11 | Additive manufacturing: A framework for implementation | Mellor, S., Hao, L., Zhang, D. | 2014 | International Journal of Production Economics 149, pp. 194-201 | Market research and environment | environment | economy | ||||
| 2017 | E12 | The impact of additive manufacturing on supply chains | Christian F. Durach, Stefan Kurpjuweit, Stephan M. Wagner | 2017 | International Journal of Physical Distribution & Logistics Management, Vol. 47 Issue: 10, pp.954-971, | Market research and environment | environment | economy | ||||
| 2017 | E13 | E-commerce channels for additive manufacturing: an exploratory study | Daniel R Eyers, Andrew T Potter | 2015 | Journal of Manufacturing Technology Management, Vol. 26 Issue: 3, pp.390-411 | Market research and environment | environment | economy | ||||
| 2017 | E14 | The role of Design for Additive Manufacturing in the successful economical introduction of AM | T.H.J. Vaneker | 2017 | Procedia CIRP 60 ( 2017 ) 181 – 186 | Market research and environment | environment | economy | ||||
| 2019 | E15 | Impact of Total Build Height and Batch Size on Environmental Performance of Electron Beam Melting | Le, V.T., Paris, H. | 2018 | Procedia CIRP 69, pp. 112-117 | Market research and environment | environment | environment | ||||
| 2019 | E16 | Framework to Combine Technical, Economic and Environmental Points of View of Additive Manufacturing Processes | Yosofi, M., Kerbrat, O., Mognol, P. | 2018 | Procedia CIRP 69, pp. 118-123 | Market research and environment | environment | DFM, environment, economy | ||||
| 2021 | E17 | Additive manufacturing and its societal impact: a literature review | Samuel H. Huang and Peng Liu and Abhiram Mokasdar and Liang Hou | 2013 | Journal Article published Jul 2013 in The International Journal of Advanced Manufacturing Technology volume 67 issue 5-8 on pages 1191 to 1203 | Market research and environment | environment | DFM, environment, economy, HEALTH | ||||
| 2021 | E18 | Predicting the future of additive manufacturing: A Delphi study on economic and societal implications of 3D printing for 2030 | Ruth Jiang and Robin Kleer and Frank T. Piller | 2017 | Technological Forecasting and Social Change Volume 117, April 2017, Pages 84-97 | Market research and environment | environment | economy | ||||
| 2021 | E19 | INFORME UNO. Análisis cualitativo del impacto de la impresión 3D en el sector médico y la reindustrialización | OPTFAIN, el Observatorio Permanente Tikoa de Fabricación Aditiva e Investigación Neoindustrial | 2016 | OPTFAIN, el Observatorio Permanente Tikoa de Fabricación Aditiva e Investigación Neoindustrial | Market research and environment | environment | economy | ||||
| 2021 | E20 | Wohlers Report 2001 | Terry Wohlers | 2001 | Wohlers Associates | Market research and environment | environment | economy | ||||
| 2021 | E21 | Wohlers Report 2012 | Terry Wohlers | 2012 | Wohlers Associates | Market research and environment | environment | economy | ||||
| 2021 | E22 | Wohlers Report 2013 | Terry Wohlers | 2013 | Wohlers Associates | Market research and environment | environment | economy | ||||
| 2021 | E23 | Wohlers Report 2014 | Terry Wohlers | 2014 | Wohlers Associates | Market research and environment | environment | economy | ||||
| 2021 | E24 | Wohlers Report 2015 | Terry Wohlers | 2015 | Wohlers Associates | Market research and environment | environment | economy | ||||
| 2021 | E25 | Wohlers Report 2016 | Terry Wohlers | 2016 | Wohlers Associates | Market research and environment | environment | economy | ||||
| 2021 | E26 | Wohlers Report 2017 | Terry Wohlers | 2017 | Wohlers Associates | Market research and environment | environment | economy | ||||
| 2021 | E27 | Wohlers Report 2018 | Terry Wohlers | 2018 | Wohlers Associates | Market research and environment | environment | economy | ||||
| 2021 | E28 | Estudio de Mercado, 3D Ingenieria BQ SAS | Javier Vargas Duque | 2016 | 3D Ingenieria BQ SAS | Market research and environment | environment | economy | ||||
| 2017 | OT1 | Reverse modelling of natural rock joints using 3D scanning and 3D printing | Quan Jiang ⇑, Xiating Feng, Yanhua Gong, Leibo Song, Shuguang Ran, Jie Cui | 2016 | Computers and Geotechnics 73 (2016) 210–220 | Other applications | another | modeling stone joints | ||||
| 2017 | OT2 | Constitutive parameter identification of 3D printing material based on the virtual fields method | Xianglu Dai, Huimin Xie | 2015 | Measurement 59 (2015) 38–43 | Other applications | another | measurement | ||||
| 2017 | OT3 | On the use of computational multi-body dynamics analysis inSLS-based 3D printing | Hammad Mazhara, Tim Osswaldb, Dan Negruta,∗ | 2016 | Additive Manufacturing xxx (2016) xxx–xxx | Other applications | another | measurement | ||||
| 2017 | OT4 | Workpiece and Machine Design in Additive Manufacturing for Multi-Axis Fused Deposition Modeling | Frederik Wullea,*, Daniel Coupeka, Florian Schäffnera, Alexander Verla, Felix Oberhoferb, Thomas Maierb | 2017 | Procedia CIRP 60 ( 2017 ) 229 – 234 | Other applications | another | machine | ||||
| 2019 | OT5 | Sensing and control in glass additive manufacturing | Peters, D., Drallmeier, J., Bristow, D.A., Landers, R.G., Kinzel, E. | 2018 | Mechatronics 56, pp. 188-197 | - | another | process control | ||||
| 2019 | OT6 | A Large Range Flexure-Based Servo System Supporting Precision Additive Manufacturing | Zhang, Z., Yan, P., Hao, G. | 2017 | Engineering 3(5), pp. 708-715 | - | another | process control |
References
[1] G. Pahl and W. Beitz, Engineering design: a systematic approach. Springer Science & Business Media, 2013.
[2] Carles Riba Romeva, Diseño Concurrente. EDICIONS UPC, 2002.
[3] L. L. Lopez Taborda, H. Maury, and J. Pacheco, “Design for additive manufacturing: a comprehensive review of the tendencies and limitations of methodologies,” Rapid Prototyp. J., vol. 27, no. 5, pp. 918–966, Jun. 2021.
[4] Luis Lisandro Lopez Taborda et al, Design methodology for Fused Filament Fabrication for medical and personalization applications: framework, Knowledge base/database, methodology, Universidad del Atlántico, Universidad del Norte, 3D Ingenieria BQ SAS, Barranquilla, Colombia, 2024.
