Summary: Medical Applications and Personalization
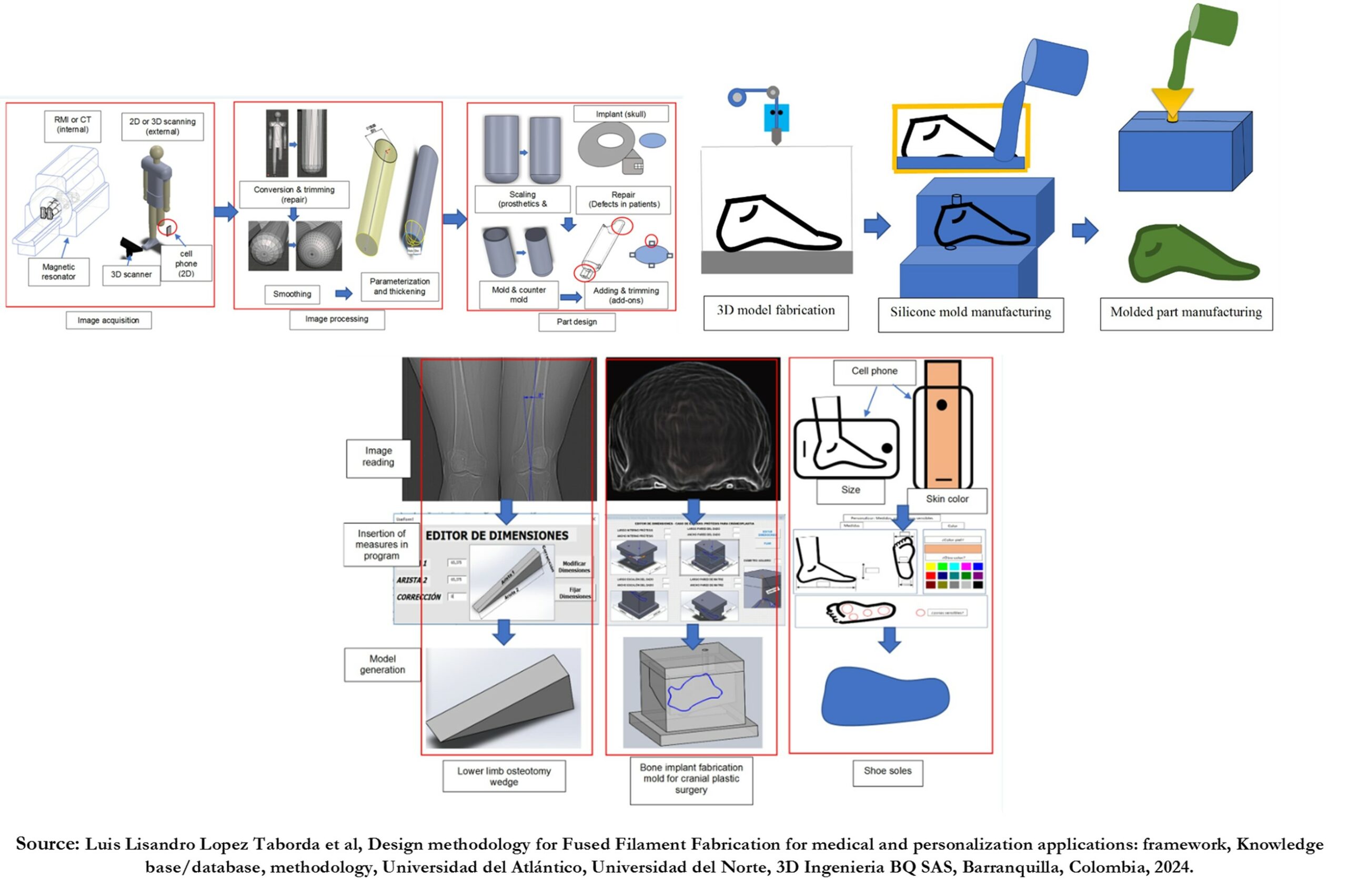
Medical and customization applications are more demanding than others due to aspects and requirements such as Individualization, mass individualization, biocompatibility and sterilization, and mechanical resistance. Each aspect is solved in a specific predefined way based on the literature.
The Extraction and Incorporation of Personalization and Individualization in the Product.
It is solved by incorporating in the design logical image information of the patient/customer that can be:
- Directly by modifying DICOM digital files such as CT, MRI, X-rays, etc. The above in case it is an internal feature of the client/patient, for example, a bone implant.
- 2D or 3D scans of the patient for features external to the client/patient, e.g., customized glasses or soles.
- Reading these files or images and incorporating the features read into the design.
In either case, computational tools that automate or facilitate the processing of files and images and design parameterization tend to reduce design and reprocessing times or even make it possible to do the process remotely online with local branches that generate and transmit the information to other locations for processing.
Personalization and Mass or Productive Individualization.
Another aspect of individualization to combat is mass production, achieved through:
- The strategy of self-service through online computerized tools of choice and selection of features for customization.
- The manufacturing by modules, from a standardized one manufactured with conventional processes with high productivity rates, but another module that interacts with the customer and is assembled to the standardized one, allows to make the production more flexible and to give an answer to standardized/customized traditional and individualized production.
Biocompatibility and Sterilization
Biocompatibility and sterilization are addressed depending on the specific type of application, for example:
- The biocompatibility of materials is not critical for medical planning and training; it is more critical for training/simulation. Aspects such as texture and hardness of tissues can be achieved by indirect fabrication with printed molds or models for flexible molds.
- As instruments and guides are not permanent implants, some materials have a sufficient degree of biocompatibility, such as PC, PEEK, or PMMA (in fact, it is material for permanent bone implants), and resistance to sterilization processes. Other materials require special silver additives, such as ABS, to be used in surgical tools, or simply the PLA impression alone sterilizes the tool if it is used freshly in surgery.
- Another way to combat biocompatibility is to use water-soluble biocompatible PCL 100 implants in cases of implants that degrade in the body without producing damage. This includes the use of continuous-release drugs to be ingested orally, using PLA containers that are biocompatible and degrade and expel naturally.
- Not manufacturing the implant directly with FFF but using a Model or Mold to manufacture parts in biocompatible materials, such as model molds for PMMA bone implants or Molds of tissue scaffolds for tissue engineering applications.
- Other implant applications are external implants, such as soft implants associated with the nose and ear or even lower prosthetic feet, which are manufactured indirectly using FFF models to manufacture molds with which the implants or prosthetic element is finally made or molds for thermoforming sheets for cosmetic covers.
Mechanical Resistance in Medical and Customized Elements
- Other designs are more demanding from the mechanical perspective, as in the case of orthoses and lower limb prostheses, where standards require resistance to specific static loads and load cycles to evaluate life. In those cases, the solution is the combination and use of several processes and materials to exceed the limits by applying the guidelines of assemblies and process chains.
- In general, keeping an open mind to possibilities and considering the final recommendations for assemblies and process chains is recommended.
